The Nautilus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Some Thoughts and Personal Opinions About Molluscan Scientific Names
A name is a name is a name: some thoughts and personal opinions about molluscan scientifi c names S. Peter Dance Dance, S.P. A name is a name is a name: some thoughts and personal opinions about molluscan scien- tifi c names. Zool. Med. Leiden 83 (7), 9.vii.2009: 565-576, fi gs 1-9.― ISSN 0024-0672. S.P. Dance, Cavendish House, 83 Warwick Road, Carlisle CA1 1EB, U.K. ([email protected]). Key words: Mollusca, scientifi c names. Since 1758, with the publication of Systema Naturae by Linnaeus, thousands of scientifi c names have been proposed for molluscs. The derivation and uses of many of them are here examined from various viewpoints, beginning with names based on appearance, size, vertical distribution, and location. There follow names that are amusing, inventive, ingenious, cryptic, ideal, names supposedly blasphemous, and names honouring persons and pets. Pseudo-names, diffi cult names and names that are long or short, over-used, or have sexual connotations are also examined. Pertinent quotations, taken from the non-scientifi c writings of Gertrude Stein, Lord Byron and William Shakespeare, have been incorporated for the benefi t of those who may be inclined to take scientifi c names too seriously. Introduction Posterity may remember Gertrude Stein only for ‘A rose is a rose is a rose’. The mean- ing behind this apparently meaningless statement, she said, was that a thing is what it is, the name invoking the images and emotions associated with it. One of the most cele- brated lines in twentieth-century poetry, it highlights the importance of names by a sim- ple process of repetition. -

Hawaiian Tree Snail Genetics
Appendix ES-9 Introduction Recent evolutionary radiations on island chains such as the Hawaiian Islands can provide insight into evolutionary processes, such as genetic drift and adaptation (Wallace 1880, Grant and Grant 1994, Losos and Ricklefs 2009). For limited mobility species, colonization processes hold important evolutionary stories not just among islands, but within islands as well (Holland and Hadfield 2002, Parent 2012). One such radiation produced at least 91 species of Hawaiian tree snails in the endemic subfamily Achatinellinae, on at least five of the six main Hawaiian Islands: O‘ahu, Maui, Lana‘i, Moloka‘i, and Hawai‘i (Pilsbry and Cooke 1912–1914, Holland and Hadfield 2007). As simultaneous hermaphrodites with the ability to self-fertilize, colonization events among islands may have occurred via the accidental transfer of a single individual by birds (Pilsbry and Cooke 1912–1914), or via land bridges that connected Maui, Molokai, and Lanai at various points in geologic history (Price and Elliot- Fisk 2004). Early naturalists attributed speciation solely to genetic drift, noting that this subfamily was “still a youthful group in the full flower of their evolution” (Pilsbry and Cooke 1912–1914). However, as these species evolved over dramatic precipitation and temperature gradients, natural selection and adaptation may have been quite rapid as species expanded to fill unexploited niches along environmental gradients, early in this subfamily’s history. As such, species in the subfamily Achatinellinae provide an excellent system for examining both neutral and adaptive processes of evolution. Habitat loss, predation by introduced species, and over-harvesting by collectors led to the extinction of more than 50 species in the subfamily Achatinellinae, and resulted in the declaration of all remaining species in the genus Achatinella as Endangered (Hadfield and Mountain 1980; U.S. -
Minutes of Discussions
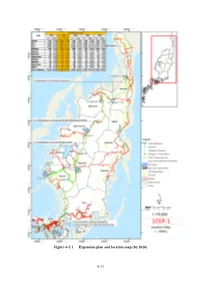
Figure 6-5.1 Expansion plan and location map (by 2020) 6-31 NGARCHELONG STATE アルコロン州 (Ollei) (Ngebei) 2.1km (Oketol) (Ngerbau) 0.9km 1.8km (Ngrill) 1.0km NGARAARD STATE ガラルド州 (Chol School) 1.0km (Urrung) 3.6km (Chelab) NGAREMLENGUI STATE NGARDMAU STATE (Ngerderemang) ガラスマオ州 1.0km アルモノグイ州 3φTr 6MW 750kVA x 1 34.5/13.8kV NGARAARD-2 S/S NGARAARD-1 S/S (Ngkeklau) 3.6km 1φTr 3x25kVA 34.5/13.8kV Busstop(Junction)-Ngardmau: 24.4km Ngardmau-Ngaraard-2: 11.8km ASAHI S/S (Ngermetengel) NGARDMAU NGIWAL STATE S/S 2.9km オギワ-ル州 3φTr 1x300kVA 34.5/13.8kV (Ogill) 1φTr 3x75kVA 34.5/13.8kV NGATPANG STATEガスパン州 IBOBANG S/S (Ngetpang Elementary School) (Ibobang) 2.0km (Ngerutoi) (Dock) (Ngetbong Ice Box) 1φTr 3x75kVA 34.5/13.8kV MELEKEOK STATE メレケオク州 AIMELIIK STATE 8.25km アイメリ-ク州 Busstop NEKKENG S/S (Junction) KOKUSAI S/S 8.8km (Ngeruling) (Oisca) 1φTr 3x75kVA 4MW 34.5/13.8kV AIMELIIK-2 S/S 3φTr 1x5MVA 6.5km 1.2km AIMELIIK-1 S/S 34.5/13.8kV (Community Center) 1φTr 3x75kVA NGCHESAR STATE 1.5km 34.5/13.8kV Busstop(Junction) – チェサ-ル州 3φTr Airai 9.0Km 1x1000kVA 34.5/13.8kV (Rai) (ELECHUI) (AIMELIIK) AIRAI S/S AIMELIIK POWER STATION アイメリ-ク発電所 N10 AIRAI STATE No.1 Tr No.2 Tr 10MVA 10MVA アイライ州 34.5/13.8kV 34.5/13.8kV 3φTr 10MVA 34.5/13.8kV (Airai State) N10 G G 6MW M6 M7 (Airport) 5MW 5MW (Mitsubishi) 15km 13.98km BABELDAOB ISLAND バベルダオブ島 K-B Bridge KOROR ISLAND コロ-ル島 Koror S/S LEGEND 凡例 3φTr PV System 10MVA 太陽光発電設備 34.5/13.8kV GENERATOR G 発電機 Malakal – Airai 9.2Km TRANSFORMER 変圧器 DISCONNECTING SWITCH 断路器 (Hechang) (Koror) LOAD BREAKER SWITCH 負荷開閉器 CIRCUIT BREAKER -

Current Affairs Q&A PDF 2019
Current Affairs Q&A PDF 2019 Current Affairs Q&A PDF 2019 Contents Current Affairs Q&A – January 2019 ..................................................................................................................... 2 INDIAN AFFAIRS ............................................................................................................................................. 2 INTERNATIONAL AFFAIRS ......................................................................................................................... 94 BANKING & FINANCE ................................................................................................................................ 109 BUSINESS & ECONOMY ............................................................................................................................ 128 AWARDS & RECOGNITIONS..................................................................................................................... 149 APPOINTMENTS & RESIGNS .................................................................................................................... 177 ACQUISITIONS & MERGERS .................................................................................................................... 200 SCIENCE & TECHNOLOGY ....................................................................................................................... 202 ENVIRONMENT ........................................................................................................................................... 215 SPORTS -

Achatinella Abbreviata (O`Ahu Tree Snail) 5-Year Review Summary And
Achatinella abbreviata (O`ahu Tree Snail) 5-Year Review Summary and Evaluation U.S. Fish and Wildlife Service Pacific Islands Fish and Wildlife Office Honolulu, Hawai`i 5-YEAR REVIEW Species reviewed: Achatinella abbreviata (O`ahu tree snail) TABLE OF CONTENTS 1.0 GENERAL INFORMATION.......................................................................................... 3 1.1 Reviewers....................................................................................................................... 3 1.2 Methodology used to complete the review:................................................................. 3 1.3 Background: .................................................................................................................. 3 2.0 REVIEW ANALYSIS....................................................................................................... 4 2.1 Application of the 1996 Distinct Population Segment (DPS) policy......................... 4 2.2 Recovery Criteria.......................................................................................................... 5 2.3 Updated Information and Current Species Status .................................................... 6 2.4 Synthesis......................................................................................................................... 9 3.0 RESULTS ........................................................................................................................ 10 3.1 Recommended Classification:................................................................................... -

BIOLOGICAL OPINION of the U.S. FISH and WILDLIFE SERVICE for ROUTINE MILITARY TRAINING and TRANSFORMATION of the 2Nd BRIGADE 25Th INFANTRY DIVISION (Light)
BIOLOGICAL OPINION of the U.S. FISH AND WILDLIFE SERVICE for ROUTINE MILITARY TRAINING and TRANSFORMATION of the 2nd BRIGADE 25th INFANTRY DIVISION (Light) U.S. ARMY INSTALLATIONS ISLAND of OAHU October 23, 2003 (1-2-2003-F-04) TABLE OF CONTENTS INTRODUCTION ........................................................... 1 CONSULTATION HISTORY .................................................. 2 BIOLOGICAL OPINION Description of the Proposed Action ............................................ 6 Dillingham Military Reservation ............................................... 11 Kahuku Training Area ..................................................... 15 Kawailoa Training Area .................................................... 20 Makua Military Reservation ................................................. 24 Schofield Barracks East Range ............................................... 25 Schofield Barracks Military Reservation ........................................ 29 South Range Acquisition Area ............................................... 35 Other Proposed SBCT Training Action Locations ................................. 36 Wildland Fire Management Plan Overview ...................................... 37 Stabilization Overview ..................................................... 38 Conservation Measures .................................................... 42 STATUS OF THE SPECIES AND ENVIRONMENTAL BASELINE - PLANTS Abutilon sandwicense ..................................................... 52 Alectryon macrococcus .................................................. -

Review of Current Wildlife Species Genetic Research: Identification of a Priority List of Wildlife Species in Trade, Where DNA Research Would Assist Law Enforcement
A Review of Current Wildlife Species Genetic Research: Identification of a priority list of wildlife species in trade, where DNA research would assist law enforcement Revised Final Report February 22, 2002 (Revised Mar 2004) Report No 3 LGC/LS/2004/001 A Review of Current Wildlife Species Genetic Research: Identification of a priority list of wildlife species in trade, where DNA research would assist law enforcement Revised Final Report Report No 3 Contact Point: Carole Foy Tel: 020 8943 7335. Prepared by: LGC: Carole Foy Lydia Ballam TRAFFIC: Crawford Allan Angela Barden Approved by: Alison Woolford ________________________________ Date: 22nd February 2002 (Revised Mar 2004) ________________________________ The work described in this report was supported under contract with DEFRA LGC/LS/2004/001 © LGC (Teddington) Limited 2004 Contents 1. Executive Summary 1 2. Project Aims 2 3. Background 2 4. Approach 5 4.1 Development of Priority Species Selection Criteria 5 4.1.1 The Primary Filter Process and Selection Criteria 5 4.1.2 Development of a DNA search strategy 8 4.2 Development of Analytical Database System 11 4.3 The Ranking, Scoring and Weighting Systems 11 4.3.1 DNA Ranking Strategy 11 5. Output 17 6. Recommendations 20 7. Conclusion 21 8. Acknowledgements 22 9. Appendices 24 9.1 Appendix 1 : Alternative animal ranking and prioritisation strategy 24 9.2 Appendix 2 : Species Database Construction and Use 26 9.3 Appendix 3 : Individuals/organisations contacted 37 9.4 Appendix 4 : Individuals/organisations offering assistance 37 9.5 Appendix 5 : DNA References 37 9.6 Appendix 6 : Summary of animal DNA information 65 9.7 Appendix 7 : Summary of plant DNA information 73 9.8 Appendix 8 : Wildlife trade regulation in the european union 75 9.9 Appendix 9 : Definitons for the Red List categories 77 Review of Current Wildlife Species Genetic Research - i - Final Report 1. -
Type Specimens of Hawaiian Land Snails in the Paleontological Research Institution in Ithaca, New York
Type Specimens of Hawaiian Land Snails in the Paleontological Research Institution in Ithaca, New York $XWKRUV*RXOGLQJ7ULFLD&6WURQJ(OOHQ(+D\HV.HQQHWK$ 6ODSFLQVN\-RKQ.LP-D\QHH5HWDO 6RXUFH$PHULFDQ0DODFRORJLFDO%XOOHWLQ 3XEOLVKHG%\$PHULFDQ0DODFRORJLFDO6RFLHW\ 85/KWWSVGRLRUJ %LR2QH&RPSOHWH FRPSOHWH%LR2QHRUJ LVDIXOOWH[WGDWDEDVHRIVXEVFULEHGDQGRSHQDFFHVVWLWOHV LQWKHELRORJLFDOHFRORJLFDODQGHQYLURQPHQWDOVFLHQFHVSXEOLVKHGE\QRQSURILWVRFLHWLHVDVVRFLDWLRQV PXVHXPVLQVWLWXWLRQVDQGSUHVVHV <RXUXVHRIWKLV3')WKH%LR2QH&RPSOHWHZHEVLWHDQGDOOSRVWHGDQGDVVRFLDWHGFRQWHQWLQGLFDWHV\RXU DFFHSWDQFHRI%LR2QH¶V7HUPVRI8VHDYDLODEOHDWZZZELRRQHRUJWHUPVRIXVH 8VDJHRI%LR2QH&RPSOHWHFRQWHQWLVVWULFWO\OLPLWHGWRSHUVRQDOHGXFDWLRQDODQGQRQFRPPHUFLDOXVH &RPPHUFLDOLQTXLULHVRUULJKWVDQGSHUPLVVLRQVUHTXHVWVVKRXOGEHGLUHFWHGWRWKHLQGLYLGXDOSXEOLVKHUDV FRS\ULJKWKROGHU %LR2QHVHHVVXVWDLQDEOHVFKRODUO\SXEOLVKLQJDVDQLQKHUHQWO\FROODERUDWLYHHQWHUSULVHFRQQHFWLQJDXWKRUVQRQSURILW SXEOLVKHUVDFDGHPLFLQVWLWXWLRQVUHVHDUFKOLEUDULHVDQGUHVHDUFKIXQGHUVLQWKHFRPPRQJRDORIPD[LPL]LQJDFFHVVWR FULWLFDOUHVHDUFK 'RZQORDGHG)URPKWWSVELRRQHRUJMRXUQDOV$PHULFDQ0DODFRORJLFDO%XOOHWLQRQ-XO 7erms of Use: https://bioone.org/terms-of-use $FFHVVSURYLGHGE\$PHULFDQ0DODFRORJLFDO6RFLHW\ Amer. Malac. Bull. 38(1): 1–38 (2020) Type specimens of Hawaiian land snails in the Paleontological Research Institution in Ithaca, New York Tricia C. Goulding1, Ellen E. Strong2, Kenneth A. Hayes1,2,3, John Slapcinsky4, Jaynee R. Kim1, and Norine W. Yeung1,2 1Malacology, Bernice Pauahi Bishop Museum, 1525 Bernice St., Honolulu, Hawaii 96817, U.S.A., [email protected] -

Ko'olau Mountains Watershed Partnership Management Plan
KO‘OLAU MOUNTAINS WATERSHED PARTNERSHIP MANAGEMENT PLAN Prepared by Jason Sumiye for The Ko‘olau Mountains Watershed Partnership Bishop Museum Dole Food Company, Inc. City and County of Honolulu Board of Water Supply Kamehameha Schools Manana Valley Farm, LLC Queen Emma Foundation State of Hawai‘i Agribusiness Development Corporation State of Hawai‘i Department of Hawaiian Home Lands State of Hawai‘i Department of Land and Natural Resources Tiana Partners, et al. U.S. Army U.S. Fish and Wildlife Service Associate Partners: State of Hawai‘i Department of Health The Nature Conservancy of Hawai‘i U.S. Environmental Protection Agency U.S. Forest Service U.S. Natural Resources Conservation Service U.S. Geological Survey Produced with the generous support of: Hawai‘i Community Foundation State of Hawai‘i Agribusiness Development Corporation State of Hawai‘i Department of Land and Natural Resources Kamehameha Schools The Nature Conservancy of Hawai‘i Copyright 2002 Cover photo taken by the U.S. Army Natural Resources Program: a view from the lower Pe‘ahināi‘a trail. Ko‘olau Mountains Watershed Partnership Management Plan EXECUTIVE SUMMARY I. INTRODUCTION................................................................................................................ 1 II. DESCRIPTION AND CURRENT CONDITION OF THE KO‘OLAU MOUNTAINS WATERSHED............................................................................................4 A. BIOPHYSICAL RESOURCES................................................................................................ -

Department of Environmental and Forest Biology
Department of Environmental and Forest Biology Department of Environmental and Forest Biology SUNY2013 -ESF - 2012 Report Annual Annual Annual Report 2012-2013 Front Cover: Collage of images provided by EFB faculty, staff, and students Department of Environmental and Forest Biology Annual Report Summer 2012 Academic Year 2012 – 2013 Donald J. Leopold Chair, Department of Environmental and Forest Biology SUNY-ESF 1 Forestry Drive Syracuse, NY 13210 Email: [email protected]; ph: (315) 470-6760 July 15, 2013 1 TABLE OF CONTENTS Introduction . 4 Overview to Annual Report . 4 New York Natural Heritage Program . 7 Building(s) . 8 Teaching . .10 Summary of main courses taught by faculty members . 10 Course teaching load summary by faculty members . 13 Undergraduate student advising loads . 15 Curriculum changes . 15 Undergraduate students enrolled in each EFB major . 15 Listing of awards and recognition . 16 Research/Scholarship . .16 Summary of publications/presentations . .16 Science Citation Indices from the Web of Science and Scopus . 16 Summary of grant activity . 18 Patents and Patent Applications . .20 Listing of awards and recognition . 20 Outreach and Service . 21 Enumeration of outreach activities . 21 Summary of grant panel service . 21 Summary of journal editorial board service. 21 Number of journal manuscripts reviewed by faculty. 22 Listing of awards and recognition . 22 Service Learning . 22 Graduate Students. 25 Number of students by degree objectives . 25 Graduate student national fellowships/awards . 25 Graduate recruitment efforts . 26 Graduate student advising . 27 Courses having TA support and enrollment in each . 28 2 Governance and Administrative Structure . .. 29 Components. 29 Supporting offices, committees, directors, and coordinators . 30 Budget . -

Checklist of Fish and Invertebrates Listed in the CITES Appendices and in EC Regulation No
JNCC Report No. 379 Checklist of fish and invertebrates listed in the CITES appendices and in EC Regulation No. 338/97 7th Edition 2005 compiled by UNEP-WCMC © JNCC 2005 The JNCC is the forum through which the three country conservation agencies - the Countryside Council for Wales, English Nature and Scottish Natural Heritage - deliver their statutory responsibilities for Great Britain as a whole, and internationally. These responsibilities contribute to sustaining and enriching biological diversity, enhancing geological features and sustaining natural systems. As well as a source of advice and knowledge for the public, JNCC is the Government's wildlife adviser, providing guidance on the development of policies for, or affecting, nature conservation in Great Britain or internationally. Published by: Joint Nature Conservation Committee Copyright: 2005 Joint Nature Conservation Committee ISBN: 1st edition published 1988 ISBN 0-86139-466-6 2nd edition published 1993 ISBN 1-873701-47-0 3rd edition published 1995 ISSN 0963-8091 4th edition published 1999 ISSN 0963-8091 5th edition published 2001 ISSN 0963-8091 6th edition published 2003 ISSN 0963-8091 7th edition published 2005 ISSN 0963-8091 Citation: UNEP-WCMC (2005). Checklist of fish and invertebrates listed in the CITES appendices and in EC Regulation 338/97. 7th Edition. JNCC Report, No. 379. Further copies of this report are available from: CITES Unit Joint Nature Conservation Committee Monkstone House City Road Peterborough PE1 1JY United Kingdom Tel: +44 1733 562626 Fax: +44 1733 555948 This document can also be downloaded from: http://www.ukcites.gov.uk and www.jncc.gov.uk Prepared under contract from the Joint Nature Conservation Committee by UNEP- WCMC.