Expression of an Ortholog of Replication Protein A1 (RPA1)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ailanthone Inhibits Non-Small Cell Lung Cancer Cell Growth Through Repressing DNA Replication Via Downregulating RPA1
FULL PAPER British Journal of Cancer (2017) 117, 1621–1630 | doi: 10.1038/bjc.2017.319 Keywords: ailanthone; non-small cell lung cancer; DNA replication; RPA1; Chinese medicine Ailanthone inhibits non-small cell lung cancer cell growth through repressing DNA replication via downregulating RPA1 Zhongya Ni1, Chao Yao1, Xiaowen Zhu1, Chenyuan Gong1, Zihang Xu2, Lixin Wang3, Suyun Li4, Chunpu Zou2 and Shiguo Zhu*,1,3 1Laboratory of Integrative Medicine, School of Basic Medical Sciences, Shanghai University of Traditional Chinese Medicine, 1200 Cai Lun Rd, Shanghai 201203, PR China; 2Department of Internal Classic of Medicine, School of Basic Medical Sciences, Shanghai University of Traditional Chinese Medicine, 1200 Cai Lun Rd, Shanghai 201203, PR China; 3Department of Immunology and Pathogenic Biology, School of Basic Medical Sciences, Shanghai University of Traditional Chinese Medicine, 1200 Cai Lun Rd, Shanghai 201203, PR China and 4Department of Pathology, School of Basic Medical Sciences, Shanghai University of Traditional Chinese Medicine, 1200 Cai Lun Rd, Shanghai 201203, PR China Background: The identification of bioactive compounds from Chinese medicine plays a crucial role in the development of novel reagents against non-small cell lung cancer (NSCLC). Methods: High throughput screening assay and analyses of cell growth, cell cycle, apoptosis, cDNA microarray, BrdU incorporation and gene expression were performed. Results: Ailanthone (Aila) suppressed NSCLC cell growth and colony formation in vitro and inhibited NSCLC tumour growth in subcutaneously xenografted and orthotopic lung tumour models, leading to prolonged survival of tumour-bearing mice. Moreover, Aila induced cell cycle arrest in a dose-independent manner but did not induce apoptosis in all NSCLC cells. -

Transcription-Replication Collisions—A Series of Unfortunate Events
biomolecules Review Transcription-Replication Collisions—A Series of Unfortunate Events Commodore St Germain 1,2,*, Hongchang Zhao 2 and Jacqueline H. Barlow 2,* 1 School of Mathematics and Science, Solano Community College, 4000 Suisun Valley Road, Fairfield, CA 94534, USA 2 Department of Microbiology and Molecular Genetics, University of California Davis, One Shields Avenue, Davis, CA 95616, USA; [email protected] * Correspondence: [email protected] (C.S.G.); [email protected] (J.H.B.) Abstract: Transcription-replication interactions occur when DNA replication encounters genomic regions undergoing transcription. Both replication and transcription are essential for life and use the same DNA template making conflicts unavoidable. R-loops, DNA supercoiling, DNA secondary structure, and chromatin-binding proteins are all potential obstacles for processive replication or transcription and pose an even more potent threat to genome integrity when these processes co-occur. It is critical to maintaining high fidelity and processivity of transcription and replication while navigating through a complex chromatin environment, highlighting the importance of defining cellular pathways regulating transcription-replication interaction formation, evasion, and resolution. Here we discuss how transcription influences replication fork stability, and the safeguards that have evolved to navigate transcription-replication interactions and maintain genome integrity in mammalian cells. Keywords: DNA replication; transcription; R-loops; replication stress Citation: St Germain, C.; Zhao, H.; Barlow, J.H. Transcription-Replication Collisions—A Series of Unfortunate Events. Biomolecules 2021, 11, 1249. 1. Introduction https://doi.org/10.3390/ From bacteria to humans, transcription has been identified as a source of genome biom11081249 instability, initially observed as spontaneous recombination referred to as transcription- associated recombination (TAR) [1]. -

Clipped Histone H3 Is Integrated Into Nucleosomes of DNA Replication
Clipped histone H3 is integrated into nucleosomes of DNA replication genes in the human malaria parasite Plasmodium falciparum Abril Marcela Herrera-solorio, Shruthi Sridhar Vembar, Cameron Ross Macpherson, Daniela Lozano-amado, Gabriela Romero Meza, Beatriz Xoconostle-cazares, Rafael Miyazawa Martins, Patty Chen, Miguel Vargas, Artur Scherf, et al. To cite this version: Abril Marcela Herrera-solorio, Shruthi Sridhar Vembar, Cameron Ross Macpherson, Daniela Lozano- amado, Gabriela Romero Meza, et al.. Clipped histone H3 is integrated into nucleosomes of DNA replication genes in the human malaria parasite Plasmodium falciparum. EMBO Reports, EMBO Press, 2019, 20 (4), pp.e46331. 10.15252/embr.201846331. hal-02321832 HAL Id: hal-02321832 https://hal.archives-ouvertes.fr/hal-02321832 Submitted on 9 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Scientific Report Clipped histone H3 is integrated into nucleosomes of DNA replication genes in the human malaria parasite -

Anti-RPA1 Monoclonal Antibody, Clone 3I21 (DCABH-3680) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Anti-RPA1 Monoclonal antibody, clone 3I21 (DCABH-3680) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Product Overview Mouse monoclonal to RPA70 Antigen Description Plays an essential role in several cellular processes in DNA metabolism including replication, recombination and DNA repair. Binds and subsequently stabilizes single-stranded DNA intermediates and thus prevents complementary DNA from reannealing.Functions as component of the alternative replication protein A complex (aRPA). aRPA binds single-stranded DNA and probably plays a role in DNA repair; it does not support chromosomal DNA replication and cell cycle progression through S-phase. In vitro, aRPA cannot promote efficient priming by DNA polymerase alpha but supports DNA polymerase delta synthesis in the presence of PCNA and replication factor C (RFC), the dual incision/excision reaction of nucleotide excision repair and RAD51-dependent strand exchange. Immunogen Purified Human RPA70 protein Isotype IgG1 Source/Host Mouse Species Reactivity Human Clone 3I21 Purity Protein G purified Conjugate Unconjugated Applications WB, IP Positive Control HeLa cells Format Liquid Size 500 μl Buffer pH: 7.40; Preservative: 0.09% Sodium azide; Constituents: 99% PBS, 0.02% BSA Preservative 0.09% Sodium Azide Storage Store at +4°C short term (1-2 weeks). Store at -20°C or -80°C. Avoid freeze / thaw cycle. 45-1 Ramsey Road, Shirley, NY 11967, USA Email: [email protected] Tel: 1-631-624-4882 Fax: 1-631-938-8221 1 © Creative Diagnostics -

CHK1 Inhibition Is Synthetically Lethal with Loss of B-Family DNA Polymerase Function in Human Lung and Colorectal Cancer Cells
Author Manuscript Published OnlineFirst on March 11, 2020; DOI: 10.1158/0008-5472.CAN-19-1372 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. 1 Title: CHK1 inhibition is synthetically lethal with loss of B-family DNA polymerase function in human lung and colorectal cancer cells. Author List: Rebecca F. Rogers1, Mike I. Walton1, Daniel L. Cherry2, Ian Collins1, Paul A. Clarke1, Michelle D. Garrett2* and Paul Workman1* Affiliations: 1Cancer Research UK Cancer Therapeutics Unit, The Institute of Cancer Research, London, SM2 5NG, UK 2 School of Biosciences, Stacey Building, University of Kent, Canterbury, Kent, CT2 7NJ, UK Running title: Synthetic lethality of CHK1 and DNA polymerase inhibition *Corresponding authors: Michelle D Garrett and Paul Workman Corresponding Author Information Michelle D Garrett School of Biosciences, Stacey Building, University of Kent, Canterbury, Kent, CT2 7NJ, UK. Email: [email protected] Phone: +44 (0)1227 816140 Paul Workman Cancer Research UK Cancer Therapeutics Unit, The Institute of Cancer Research, London, SM2 5NG, UK. Email: [email protected] Phone: +44 (0)20 7153 5209 Conflict of interest statement: IC, MDG, MIW, RFR, PC and PW are current or past employees of The Institute of Cancer Research, which has a commercial interest in the discovery and development of CHK1 inhibitors, including SRA737, and operates a rewards-to- inventors scheme. IC, MDG and MIW have been involved in a commercial collaboration on CHK1 inhibitors with Sareum Ltd and intellectual property arising from the program, including SRA737, was licensed to Sierra Oncology. IC is a consultant for Sierra Oncology, Adorx Ltd, Epidarex LLP and Enterprise Therapeutics Ltd and holds equity in Monte Rosa Therapeutics AG. -

Monitoring Replication Protein a (RPA) Dynamics in Homologous
Marquette University e-Publications@Marquette Biological Sciences Faculty Research and Biological Sciences, Department of Publications 9-19-2017 Monitoring Replication Protein A (RPA) Dynamics in Homologous Recombination Through Site-specific ncorI poration of Non- canonical Amino Acids Nilisha Pokhrel Marquette University Sofia Origanti Marquette University, [email protected] Eric Parker Davenport Marquette University Disha M. Gandhi Marquette University Kyle Kaniecki Columbia University See next page for additional authors Published version. Nucleic Acids Research, Vol. 45, No. 16 (September 19, 2017): 9413-9426. DOI. Authors Nilisha Pokhrel, Sofia Origanti, Eric Parker Davenport, Disha M. Gandhi, Kyle Kaniecki, Ryan A. Mehl, Eric C. Greene, Chris Dockendorff, and Edwin Antony This article is available at e-Publications@Marquette: https://epublications.marquette.edu/bio_fac/603 Published online 12 July 2017 Nucleic Acids Research, 2017, Vol. 45, No. 16 9413–9426 doi: 10.1093/nar/gkx598 Monitoring Replication Protein A (RPA) dynamics in homologous recombination through site-specific incorporation of non-canonical amino acids Nilisha Pokhrel1, Sofia Origanti1, Eric Parker Davenport1, Disha Gandhi2, Kyle Kaniecki3,4, Ryan A. Mehl5, Eric C. Greene3, Chris Dockendorff2 and Edwin Antony1,* 1Department of Biological Sciences, Marquette University, Milwaukee, WI 53201, USA, 2Department of Chemistry, Marquette University, Milwaukee, WI 53201, USA, 3Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY -

Identification of Proteins Involved in the Maintenance of Genome Stability
Identification of Proteins Involved in the Maintenance of Genome Stability by Edith Hang Yu Cheng A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Department of Biochemistry University of Toronto ©Copyright by Edith Cheng2015 Identification of Proteins Involved in the Maintenance of Genome Stability Edith Cheng Doctor of Philosophy Department of Biochemistry University of Toronto 2015 Abstract Aberrant changes to the genome structure underlie numerous human diseases such as cancers. The functional characterization ofgenesand proteins that maintain chromosome stability will be important in understanding disease etiology and developing therapeutics. I took a multi-faceted approach to identify and characterize genes involved in the maintenance of genome stability. As biological pathways involved in genome maintenance are highly conserved in evolution, results from model organisms can greatly facilitate functional discovery in humans. In S. cerevisiae, I identified 47 essential gene depletions with elevated levels of spontaneous DNA damage foci and 92 depletions that caused elevated levels of chromosome rearrangements. Of these, a core subset of 15 DNA replication genes demonstrated both phenotypes when depleted. Analysis of rearrangement breakpoints revealed enrichment at yeast fragile sites, Ty retrotransposons, early origins of replication and replication termination sites. Together, thishighlighted the integral role of DNA replicationin genome maintenance. In light of my findings in S. cerevisiae, I identified a list of 153 human proteins that interact with the nascentDNA at replication forks, using a DNA pull down strategy (iPOND) in human cell lines. As a complementary approach for identifying human proteins involved in genome ii maintenance, I usedthe BioID techniqueto discernin vivo proteins proximal to the human BLM- TOP3A-RMI1-RMI2 genome stability complex, which has an emerging role in DNA replication progression. -

DNA Unwinding by Replication Protein a Is a Property of the 32 Kda Subunit
1993 Oxford University Press Nucleic Acids Research, 1993, Vol. 21, No. 16 3659-3665 DNA unwinding by replication protein A is a property of the 70 kDa subunit and is facilitated by phosphorylation of the 32 kDa subunit Anthi Georgaki and Ulrich Hubscher* Department of Veterinary Biochemistry, University of Zurich-lrchel, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland Received May 28, 1993; Revised and Accepted July 1, 1993 ABSTRACT Replication protein A (RP-A) is a heterotrimeric single- three subunits of 70 kDa, 32-34 kDa and 11-14 kDa and binds stranded DNA binding protein with important functions preferentially to ssDNA through its 70 kDa subunit. RP-A in DNA replication, DNA repair and DNA recombination. appears to have an important role in the T antigen dependent We have found that RP-A from calf thymus can unwind generation of a ss region at the SV40 origin prior to initiation DNA in the absence of ATP and MgCI2, two essential of DNA synthesis. This protein is highly conserved since it has cofactors for bona fide DNA helicases (Georgaki, A., been found to have a similar subunit structure and function in Strack, B., Podust, V. and Hubscher, U. FEBS Lett. 308, Saccharomyces cerevisiae [3]. RP-A has a preference to bind 240 - 244, 1992). DNA unwinding by RP-A was found to ss pyrimidine stretches in a stoichiometry of 1 molecule to to be sensitive to MgCI2, ATP, heating and freezing/ 30 bases [2]. In addition, it has direct effects on DNA thawing. Escherichia coil single stranded DNA binding polymerases a and a [4]. -

Replication Protein a Is an Independent Prognostic Indicator with Potential Therapeutic Implications in Colon Cancer
Modern Pathology (2007) 20, 159–166 & 2007 USCAP, Inc All rights reserved 0893-3952/07 $30.00 www.modernpathology.org Replication protein A is an independent prognostic indicator with potential therapeutic implications in colon cancer Nikolaos Givalos1, Hariklia Gakiopoulou2, Melina Skliri2, Katerina Bousboukea2, Anastasia E Konstantinidou2, Penelope Korkolopoulou2, Maria Lelouda2, Gregory Kouraklis1, Efstratios Patsouris2 and Gabriel Karatzas1 1Department of Surgery, Medical School, National Kapodistrian University of Athens, Athens, Greece and 2Department of Pathology, Medical School, National Kapodistrian University of Athens, Athens, Greece Replication protein A (RPA), a component of the origin recognition complex, is required for stabilization of single-stranded DNA at early and later stages of DNA replication being thus critical for eukaryotic DNA replication. Experimental studies in colon cancer cell lines have shown that RPA protein may be the target of cytotoxins designed to inhibit cellular proliferation. This is the first study to investigate the expression of RPA1 and RPA2 subunits of RPA protein and assess their prognostic value in colon cancer patients. We analyzed immunohistochemically the expression of RPA1 and RPA2 proteins in a series of 130 colon cancer resection specimens in relation to conventional clinicopathological parameters and patients’ survival. Statistical significant positive associations emerged between: (a) RPA1 and RPA2 protein expressions (P ¼ 0.0001), (b) RPA1 and RPA2 labelling indices (LIs) and advanced stage of the disease (P ¼ 0.001 and 0.003, respectively), (c) RPA1 and RPA2 LIs and the presence of lymph node metastasis (P ¼ 0.002 and 0.004, respectively), (d) RPA1 LI and the number of infiltrated lymph nodes (P ¼ 0.021), (e) RPA2 LI and histological grade of carcinomas (P ¼ 0.05). -

A Conserved but Plant-Specific CDK-Mediated Regulation of DNA Replication Protein A2 in the Precise Control of Stomatal Terminal Division
A conserved but plant-specific CDK-mediated regulation of DNA replication protein A2 in the precise control of stomatal terminal division Kezhen Yanga, Lingling Zhua,b, Hongzhe Wanga, Min Jianga, Chunwang Xiaoc,d, Xiangyang Hue, Steffen Vannestef,g,h, Juan Dongi, and Jie Lea,b,1 aKey Laboratory of Plant Molecular Physiology, CAS Center for Excellence in Molecular Plant Sciences, Institute of Botany, Chinese Academy of Sciences, 100093 Beijing, China; bUniversity of Chinese Academy of Sciences, 100049 Beijing, China; cCollege of Life and Environmental Sciences, Minzu University of China, 100081 Beijing, China; dHulun Lake Reserve Grassland Ecology Research Station, Minzu University of China, 100081 Beijing, China; eShanghai Key Laboratory of Bio-Energy Crops, School of Life Sciences, Shanghai University, 200444 Shanghai, China; fCenter for Plant Systems Biology, VIB, 9052 Ghent, Belgium; gDepartment of Plant Biotechnology and Bioinformatics, Ghent University, 9052 Ghent, Belgium; hLaboratory of Plant Growth Analysis, Ghent University Global Campus, 21985 Incheon, Republic of Korea; and iWaksman Institute of Microbiology, Rutgers, The State University of New Jersey, Piscataway, NJ 08854 Edited by David C. Baulcombe, University of Cambridge, Cambridge, United Kingdom, and approved July 29, 2019 (received for review November 11, 2018) The R2R3-MYB transcription factor FOUR LIPS (FLP) controls the condition, RPA2 is hyperphosphorylated by the PIKK-family stomatal terminal division through transcriptional repression of kinases (ATM, ATR, and DNA-PK) that facilitates mitotic exit the cell cycle genes CYCLIN-DEPENDENT KINASE (CDK) B1s (CDKB1s), and the initiation of DNA repair (13–15). All known RPA2 CDKA;1,andCYCLIN A2s (CYCA2s). We mutagenized the weak mu- homologs have a conserved N-terminal phosphorylation domain, tant allele flp-1 seeds with ethylmethane sulfonate and screened although the specific residues may be not conserved in different out a flp-1 suppressor 1 (fsp1) that suppressed the flp-1 stomatal species (11). -
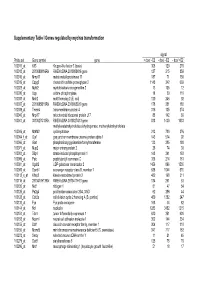
Supplementary Table I Genes Regulated by Myc/Ras Transformation
Supplementary Table I Genes regulated by myc/ras transformation signal Probe set Gene symbol gene + dox/ - E2 - dox/ - E2 - dox/ +E2 100011_at Klf3 Kruppel-like factor 3 (basic) 308 120 270 100013_at 2010008K16Rik RIKEN cDNA 2010008K16 gene 127 215 859 100016_at Mmp11 matrix metalloproteinase 11 187 71 150 100019_at Cspg2 chondroitin sulfate proteoglycan 2 1148 342 669 100023_at Mybl2 myeloblastosis oncogene-like 2 13 105 12 100030_at Upp uridine phosphorylase 18 39 110 100033_at Msh2 mutS homolog 2 (E. coli) 120 246 92 100037_at 2310005B10Rik RIKEN cDNA 2310005B10 gene 178 351 188 100039_at Tmem4 transmembrane protein 4 316 135 274 100040_at Mrpl17 mitochondrial ribosomal protein L17 88 142 89 100041_at 3010027G13Rik RIKEN cDNA 3010027G13 gene 818 1430 1033 methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate 100046_at Mthfd2 cyclohydrolase 213 720 376 100064_f_at Gja1 gap junction membrane channel protein alpha 1 143 574 92 100066_at Gart phosphoribosylglycinamide formyltransferase 133 385 180 100071_at Mup2 major urinary protein 2 26 74 30 100081_at Stip1 stress-induced phosphoprotein 1 148 341 163 100089_at Ppic peptidylprolyl isomerase C 358 214 181 100091_at Ugalt2 UDP-galactose translocator 2 1456 896 1530 100095_at Scarb1 scavenger receptor class B, member 1 638 1044 570 100113_s_at Kifap3 kinesin-associated protein 3 482 168 311 100116_at 2810417H13Rik RIKEN cDNA 2810417H13 gene 134 261 53 100120_at Nid1 nidogen 1 81 47 54 100125_at Pa2g4 proliferation-associated 2G4, 38kD 62 286 44 100128_at Cdc2a cell division cycle 2 homolog A (S. pombe) 459 1382 247 100133_at Fyn Fyn proto-oncogene 168 44 82 100144_at Ncl nucleolin 1283 3452 1215 100151_at Tde1 tumor differentially expressed 1 620 351 620 100153_at Ncam1 neural cell adhesion molecule 1 302 144 234 100155_at Ddr1 discoidin domain receptor family, member 1 304 117 310 100156_at Mcmd5 mini chromosome maintenance deficient 5 (S. -

DNA Repair Deficiency in Huntington's Disease Fibroblasts and Induced Pluripotent Stem Cells
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Fall 2015 DNA Repair Deficiency in Huntington's Disease Fibroblasts and Induced Pluripotent Stem Cells Peter Anthony Mollica Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Cell Biology Commons, Developmental Biology Commons, and the Molecular Biology Commons Recommended Citation Mollica, Peter A.. "DNA Repair Deficiency in Huntington's Disease Fibroblasts and Induced Pluripotent Stem Cells" (2015). Doctor of Philosophy (PhD), Dissertation, Biological Sciences, Old Dominion University, DOI: 10.25777/r105-c396 https://digitalcommons.odu.edu/biology_etds/7 This Dissertation is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. DNA REPAIR DEFICIENCY IN HUNTINGTON’S DISEASE FIBROBLASTS AND INDUCED PLURIPOTENT STEM CELLS by Peter A. Mollica B.S. December 2011, Old Dominion University A Dissertation Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY BIOMEDICAL SCIENCES OLD DOMINION UNIVERSITY December 2015 Approved by : ______________________________ Christopher J. Osgood (Co-Advisor) ______________________________ Roy C. Ogle (Co-Advisor) ______________________________ Lesley H. Greene (Member) ABSTRACT DNA REPAIR DEFICIENCY IN HUNTINGTON’S DISEASE FIBROBLASTS AND INDUCED PLURIPOTENT STEM CELLS Peter A. Mollica Old Dominion University, 2015 Co-Advisors: Dr. Christopher Osgood Dr. Roy C. Ogle Mutant huntingtin protein (mhtt)–the protein responsible for cellular dysfunction in Huntington’s disease (HD) –is a product of an expanded trinucleotide repeat (TNR) cytosine-adenine-guanine (CAG) sequence in exon 1 of the huntingtin (HTT) gene.