Rapid Testing of Salinity Effects on Pistachio Seedling Rootstocks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
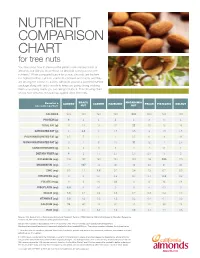
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Health Benefits of Pistachio Consumption in Pre-Diabetic Subjects
HEALTH BENEFITS OF PISTACHIO CONSUMPTION IN PRE-DIABETIC SUBJECTS Pablo Hernández Alonso ADVERTIMENT. L'accés als continguts d'aquesta tesi doctoral i la seva utilització ha de respectar els drets de la persona autora. Pot ser utilitzada per a consulta o estudi personal, així com en activitats o materials d'investigació i docència en els termes establerts a l'art. 32 del Text Refós de la Llei de Propietat Intel·lectual (RDL 1/1996). Per altres utilitzacions es requereix l'autorització prèvia i expressa de la persona autora. En qualsevol cas, en la utilització dels seus continguts caldrà indicar de forma clara el nom i cognoms de la persona autora i el títol de la tesi doctoral. No s'autoritza la seva reproducció o altres formes d'explotació efectuades amb finalitats de lucre ni la seva comunicació pública des d'un lloc aliè al servei TDX. Tampoc s'autoritza la presentació del seu contingut en una finestra o marc aliè a TDX (framing). Aquesta reserva de drets afecta tant als continguts de la tesi com als seus resums i índexs. ADVERTENCIA. El acceso a los contenidos de esta tesis doctoral y su utilización debe respetar los derechos de la persona autora. Puede ser utilizada para consulta o estudio personal, así como en actividades o materiales de investigación y docencia en los términos establecidos en el art. 32 del Texto Refundido de la Ley de Propiedad Intelectual (RDL 1/1996). Para otros usos se requiere la autorización previa y expresa de la persona autora. En cualquier caso, en la utilización de sus contenidos se deberá indicar de forma clara el nombre y apellidos de la persona autora y el título de la tesis doctoral. -

Health and Nutrition Research
Health and Nutrition Research Pistachios can help individuals maintain good health, support an active lifestyle and reduce the risk of nutrition-related diseases. Research studies suggest that pistachios have numerous health benefits, including being a source of health-boosting antioxidants and other important nutrients, lowering the risk of heart disease, supporting weight management and a healthy diet, creating a lower-than-expected blood-sugar level and helping with insulin sensitivity. Subjects who ate more than three servings of nuts (such as pistachios) per week had a 39% lower mortality risk. The PREDIMED Study. Guasch-Ferré, et al. BMC medicine 2013Jul16; 11:164 AmericanPistachios.org Pistachios and Mortality Many large population studies have found an inverse association between nut intake and total mortality. Recently published in the British Journal of Nutrition, nut intake was associated with a lower risk of all-cause mortality in a large prospective study of 19,386 participants. As compared with subjects who did not eat nuts, those who consumed nuts more than 8 times per month showed a 47% lower risk of dying from any cause.1 Similar results have been found in studies from populations around the world.2 An analysis of studies for all-cause, cancer and cardiovascular disease (CVD) mortality, with a total of 354,933 participants, nut consumption was associated with significant protection. One-serving of nuts per day resulted in 27% lower risk from dying from any cause including CVD and cancer.3 In another systematic review and analysis of large, well-designed prospective population studies in Europe and North America showed that nut consumption is inversely associated with all-cause mortality, total CVD mortality, coronary heart disease mortality and sudden cardia death. -

To See a List of Possible Ice Cream Choices
After Dinner Mint Almond Almond Crisp (w peanuts and rice cereal) Almond Delight Almond Linzertorte (w raspberry jam) Almond Poppy Seed Ambrosia (Banana Ice Cream w coconut, orange and almonds) Anise Apple Brown Betty (w ginger snaps) Apple Butter Apple Cheddar Apple Cherry Apple Cinnamon Coffee Cake Apple Pie Apple Raisin Walnut Apple Strawberry Apple Thyme Applesauce Apricot Apricot Almond Apricot Jam Apricot Orange Asia Spice (Green Tea ice cream w szechuan peppercorns) Autumn (Nutmeg ice cream w prunes, dates & figs) Avocado Aztec "Hot" Chocolate (Chocolate w chile powder) Baked Apple Balsamic Caramel (w balsamic vinegar) Banana Banana Candy Bar Banana Carob Chip Banana Chocolate Chip Banana Coconut Banana Cookie Banana Cream Pie Banana Fudge Banana Fudge Chunk Banana Malt Banana Marshmellow Banana Nut Banana Orange Banana Peanut Butter Banana Philadelphia Style ( w/o eggs) Banana Strawberry Banana Tart Banana w Caramelized White Chocolate Freckles Bangkok Peanut Beet w Mascarpone, Orange Zest & Poppy Seeds Basil Page 1 Beet w Mascarpone, Orange Zest & Poppy Seeds Berry Crisp Birthday Cake Biscuit Tortoni Bittersweet Chocolate-Laced Vanilla Black Coffee Black Currant Tea Black Pepper Black Pine (Pine Nut ice cream w black licorice candy) Black Walnut Blackberry Blackberry Jam Blackstrap Praline (w blackstrap molasses) Blueberry Blueberry Jam Blueberry Lemon Sour Cream Brown Bread Brown Butter Almond Brittle Bubble Gum Burnt Almond Burnt Sugar Burnt Sugar Pie Burnt Walnut Butter Cake, Gooey Butter Fruitcake Butter Pecan Butter w Honey -

Raw and Roasted Pistachio Nuts (Pistacia Vera L.) Are ‘Good’ Sources of Protein Based on Their Digestible Indispensable Amino Acid Score As Determined in Pigs
Research Article Received: 12 September 2019 Revised: 13 April 2020 Accepted article published: 23 April 2020 Published online in Wiley Online Library: 19 May 2020 (wileyonlinelibrary.com) DOI 10.1002/jsfa.10429 Raw and roasted pistachio nuts (Pistacia vera L.) are ‘good’ sources of protein based on their digestible indispensable amino acid score as determined in pigs Hannah M Bailey and Hans H Stein* Abstract BACKGROUND: Pistachio nuts may be consumed as raw nuts or as roasted nuts. However, there is limited information about the protein quality of the nuts, and amino acid (AA) digestibility and protein quality have not been reported. Therefore, the objec- tive of this research was to test the hypothesis that raw and roasted pistachio nuts have a digestible indispensable AA score (DIAAS) and a protein digestibility corrected AA score (PDCAAS) greater than 75, thereby qualifying them as a good source of protein. RESULTS: The standardized ileal digestibility (SID) of all indispensable AAs, except arginine and phenylalanine, was less in roasted pistachio nuts than in raw pistachio nuts (P < 0.05). Raw pistachio nuts had a PDCAAS of 73, and roasted pistachio nuts had a PDCAAS of 81, calculated for children 2–5 years, and the limiting AA in the PDCAAS calculation was threonine. The DIAAS values calculated for children older than 3 years, adolescents, and adults was 86 and 83 for raw and roasted pistachio nuts respectively. The limiting AA in both raw and roasted pistachio nuts that determined the DIAAS for this age group was lysine. CONCLUSION: The results of this research illustrate that raw and roasted pistachio nuts can be considered a good quality pro- tein source with DIAAS greater than 75; however, processing conditions associated with roasting may decrease the digestibility of AAs in pistachio nuts. -

Growing Pistachios in New Mexico
Growing Pistachios in New Mexico IC EX O S M T A W T E E Cooperative Extension Service • Circular 532 N U College of Agriculture and Home Economics N Y I IT VERS Growing Pistachios In New Mexico Esteban Herrera, Extension Horticulturist Public interest in pistachio cultivation has increased peratures above 100°F are described as ideal. Pistachio in New Mexico over the past five years. Pistachio nuts trees thrive on heat; better nut filling and less blanks are produced in the state seem to be of excellent quality, produced in hot-weather climates. However, winters suggesting the crop may have a commercial future in need to be cold enough to complete their dormancy (a southern New Mexico. rest period during winter). Three-leaflet leaves are pro- duced instead of the normal five-leaflet leaves when cold requirements are not met. About 1,000 accumu- HISTORY lated hours of temperatures at 45°F or below are required for pistachio trees to break dormancy, and to start There are about 11 species of pistachio trees (Pistacia normal growth in the spring. Trees should not be planted spp. L). P. vera is the only species grown commercially above 4,500 feet elevation because cool summer tem- because it produces fruit of adequate size to be mar- peratures do not promote good kernel development. keted. Species such as P. atlantica, P. terebinthus and Also, temperatures below 10°F can kill the tree, espe- P. integerrima are used as rootstocks for P. vera. The cially young trees. P. terebinthus is more resistant to pistachio’s origin is still uncertain, but most experts cold than P. -

British Journal of Nutrition (2015), 113, S79–S93 Doi:10.1017/S0007114514003250 Q the Authors 2015
Downloaded from British Journal of Nutrition (2015), 113, S79–S93 doi:10.1017/S0007114514003250 q The Authors 2015 https://www.cambridge.org/core q Nutrition attributes and health effects of pistachio nuts M. Bullo´1,2*, M. Juanola-Falgarona1,2, P. Herna´ndez-Alonso1 and J. Salas-Salvado´1,2* 1 Human Nutrition Unit, Hospital Universitari de Sant Joan de Reus, Faculty of Medicine and Health Sciences, . IP address: IISPV (Institut d’Investigacio´ Sanita`ria Pere Virgili), Universitat Rovira i Virgili, C/Sant Llorenc¸ 21, 43201 Reus, Spain 2CIBERobn (Centro de Investigacio´n Biome´dica en Red Fisiopatologı´a de la Obesidad y Nutricio´n), Institute of Health Carlos III, Madrid, Spain 170.106.35.234 (Submitted 19 June 2014 – Final revision received 3 September 2014 – Accepted 4 September 2014) , on Abstract 28 Sep 2021 at 08:13:30 Epidemiological and/or clinical trials have suggested that nut consumption has a beneficial impact on health outcomes such as hypertension, diabetes, CVD, cancer, other inflammatory conditions and total mortality. Nuts are nutrient-dense foods with a healthy fatty acid profile, as well as provide other bioactive compounds with recognised health benefits. Among nuts, pistachios have a lower fat and energy content and the highest levels of K, g-tocopherol, vitamin K, phytosterols, xanthophyll carotenoids, certain minerals (Cu, Fe and Mg), vitamin B6 and thiamin. Pistachios have a high antioxidant and anti-inflammatory potential. The aforementioned characteristics and nutrient mix probably contribute to the growing body of evidence that consumption of pistachios improves health. , subject to the Cambridge Core terms of use, available at The present review examines the potential health effects of nutrients and phytochemicals in pistachios, as well as epidemiological and clinical evidence supporting these health benefits. -

Descriptive Analysis of Black Walnut Cultivars and Relationship Between Consumer Acceptance and Descriptive Analysis of Black Walnuts in a Sugar Cookie Base
DESCRIPTIVE ANALYSIS OF BLACK WALNUT CULTIVARS AND RELATIONSHIP BETWEEN CONSUMER ACCEPTANCE AND DESCRIPTIVE ANALYSIS OF BLACK WALNUTS IN A SUGAR COOKIE BASE by ASHLEY E. MILLER B.S.F.C.S., University of Georgia, 2009 A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Interdisciplinary Food Science Graduate Program College of Human Ecology KANSAS STATE UNIVERSITY Manhattan, Kansas 2012 Approved by: Major Professor Dr. Delores Chambers Copyright ASHLEY E. MILLER 2012 Abstract Researchers evaluated the flavor characteristics of seven black walnut (Juglans nigra L.) cultivars: Brown Nugget, Davidson, Emma K, Football, Sparks 127, Sparrow, and Tomboy using descriptive sensory analysis. Seven trained panelists developed a lexicon for the black walnuts and scored the intensities of the samples for 22 flavor and taste attributes. Results showed that the 7 samples differed significantly (P ≤ 0.05) on 13 of the attributes. For the majority of the attributes, only Emma K differed from the rest of the cultivars by being characterized with lower scores for black walnut ID, overall nutty, nutty-grain-like, nutty-buttery, floral/fruity, oily, and overall sweet. It also was higher in acrid, burnt, fruity-dark, musty/earthy, rancid, and bitter attributes. Researchers then incorporated the black walnut cultivars into a simple cookie recipe and evaluated the samples for differences in flavor attributes using the same trained panelists. Nine of the 25 attributes differed significantly across cultivars: black walnut ID, overall nutty, nutty-buttery, brown, toasted, acrid, rancid, overall sweet, and sweet (P ≤ 0.05). Lower mean scores in black walnut ID, overall nutty, and sweet and higher mean scores in rancid and acrid characterized the Emma K cookie. -
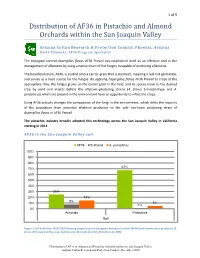
2020 Report on the Distribution of AF36 in Pistachio and Almond
1 of 5 Distribution of AF36 in Pistachio and Almond Orchards within the San Joaquin Valley Arizona Cotton Research & Protection Council, Phoenix, Arizona David Edmunds, AF36 Program Specialist The biological control Aspergillus flavus AF36 Prevail has established itself as an effective tool in the management of aflatoxins by using a native strain of the fungus incapable of producing aflatoxins. The beneficial strain, AF36, is coated onto a carrier grain that is sterilized, meaning it will not germinate, and serves as a food source for the fungus. By applying Aspergillus flavus AF36 Prevail to crops at the appropriate time, the fungus grows on the carrier grain in the field, and its spores move to the desired crop by wind and insects before the aflatoxin-producing strains (A. flavus S-morphotype and A. parasiticus) which are present in the environment have an opportunity to infect the crops. Using AF36 actually changes the composition of the fungi in the environment, which shifts the majority of the population from potential aflatoxin producers to the safe non-toxin producing strain of Aspergillus flavus in AF36 Prevail. The pistachio industry broadly adopted this technology across the San Joaquin Valley in California starting in 2012. AF36 in the San Joaquin Valley soil AF36 S-strains A. parasiticus 100% 90% 80% 67% 70% 60% 50% 40% 30% 25% 20% 14% 8% 10% 5% 1% 0% Almonds Pistachios Soil Figure 1 Soil data from 2018-2020 showing proportions of atoxigenic biological control (AF36) and known toxin-producers (S- strain, AP) separated by crop. Sample size: Almonds (n=237), Pistachios (n=390). -

Harvesting and Storing Your Home Orchard's Nut Crop
PUBLICATION 8005 Harvesting and Storing Your Home Orchard’s Nut Crop: Almonds, Walnuts, Pecans, Pistachios, and Chestnuts UNIVERSITY OF CALIFORNIA ED PERRY, University of California Cooperative Extension Farm Advisor, Division of Agriculture Stanislaus County; G. STEVEN SIBBETT, University of California Cooperative Extension and Natural Resources Farm Advisor, Tulare County. http://danrcs.ucdavis.edu he quality and quantity of your home orchard’s nut crop depend on the harvest- Ting, processing, and storage techniques that you use. This publication offers advice for handling almonds, chestnuts, pecans, pistachios, and walnuts. GENERAL TIPS When to Harvest Harvest all types of nuts as soon as they are ready. Late harvesting reduces crop volume, lowers nut quality, and shortens storage life. In California, harvest timing varies somewhat with location. The earliest harvest generally occurs in the southern San Joaquin Valley, followed by the central San Joaquin Valley, the Sacramento Valley, and finally the coastal valleys. An interval of about seven days separates the begin- ning dates for harvest in each succeeding district. Harvesting To harvest nuts (except chestnuts), knock or shake them from their trees with long wooden, fiberglass, or plastic (PVC) poles. You can knock almonds from the trees using rubber mallets or poles that are available from farm supply stores. CAUTION: When harvesting nuts with a pole, use extreme caution. Any contact between the pole and power lines will result in serious injury or death. Do not use aluminum or other metal poles to harvest nuts: these are especially hazardous around power lines. Loosened nuts often follow the pole to the ground, striking the person holding the pole. -

Sensory Profiles and Seasonal Variation of Black Walnut Cultivars and the Relationship Between Sensory Characteristics and Consumer Acceptance of Black Walnut Gelato
SENSORY PROFILES AND SEASONAL VARIATION OF BLACK WALNUT CULTIVARS AND THE RELATIONSHIP BETWEEN SENSORY CHARACTERISTICS AND CONSUMER ACCEPTANCE OF BLACK WALNUT GELATO by CATHERINE A. LYNCH B.S., Iowa State University, 2012 A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Food Science KANSAS STATE UNIVERSITY Manhattan, Kansas 2015 Approved by: Major Professor Dr. Kadri Koppel Abstract Black walnut (Juglans nigra L.) is a Juglans species native to the United States. Nuts are collected each fall from black walnut trees and the kernels are consumed in many food products like ice cream, candies, and baked goods. Flavor profiles of black walnut cultivars have been examined, but no studies have looked at the effect of growing season on flavor profile, and few studies have determined consumer acceptance of black walnut food products. The sensory profiles of 10 black walnut cultivars (Football, Vandersloot, Brown Nugget, Pounds, Sparks 127, Davidson, Sparrow, Neel, Emma K, and Tomboy) were evaluated using descriptive sensory analysis. A trained panel scored the intensity of 3 appearance, 7 aroma, 23 flavor, and 6 texture attributes. Results showed that the cultivars differed significantly (P≤0.05) on 11 of these attributes. The results from this study were also compared to results collected in 2011 of 7 black walnut cultivars. Two flavor attributes (black walnut ID and overall nutty) had an interaction effect of year and cultivar, while 7 attributes showed a main effect of year (brown, caramelized, floral/fruity, fruity,-dark piney, musty/dusty, and oily). In general, flavor attributes had higher intensities in 2011 than in 2013. -

International Aflatoxin Tolerances
industry backgrounder • october 2016 International Aflatoxin Tolerances COUNTRY TOLERANCE COMMENTS µg/kg (PPB) Algeria 8 Total Nuts, cereal Argentina 20 Total All foodstuffs Austria See EU Australia 15 Total Nuts Bahrain 20 Total GCC Regulations .05 Total Children and infant food Belgium See EU Bolivia 20 Total Bosnia & Herzegovina 8 B1 For direct human consumption 10 Total For direct human consumption 12 B1 For further processing 15 Total For further processing Brazil 10 Total Bulgaria See EU Canada 15 Total Calculated on the nut meat portion Chile 10 Total China 5 B1 20 Total CODEX 15 Total Colombia* 10 Total Costa Rica 20 Total Croatia See EU Cyprus See EU Cuba 5 Total Czech Republic See EU Denmark See EU Egypt See EU El Salvador 15 Total Further processing 10 Total Ready to eat Estonia See EU Ethiopia 15 Total Codex EU 27: 10 Total (8 B1) Almonds and pistachios ready to eat COUNTRY TOLERANCE COMMENTS µg/kg (PPB) Austria, Belgium, Bulgaria, 4 Total (2 B1) Peanuts ready to eat Cyprus, Czech Republic, Denmark, Estonia, Finland, 10 Total (5 B1) Hazelnuts and brazil nuts ready to eat France, Germany, Greece, Hungary, Ireland, Italy, 15 Total (12 B1) Almonds and pistachios for further Latvia, Lithuania, processing Luxembourg, Malta, Netherlands, Poland, 15 Total (8 B1) Peanuts, hazelnuts, and brazil nuts for Portugal, Romania, further processing Slovakia, Slovenia, Spain, Sweden, and the UK Finland See EU France See EU Germany See EU Greece See EU Guatemala 15 Total Codex Honduras 15 Total Further processing 10 Total Ready to eat Hong Kong