Riffle Snaketail & Endangered Species Ophiogomphus Carolus Program
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Olive Clubtail (Stylurus Olivaceus) in Canada, Prepared Under Contract with Environment Canada
COSEWIC Assessment and Status Report on the Olive Clubtail Stylurus olivaceus in Canada ENDANGERED 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Olive Clubtail Stylurus olivaceus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 58 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC would like to acknowledge Robert A. Cannings, Sydney G. Cannings, Leah R. Ramsay and Richard J. Cannings for writing the status report on Olive Clubtail (Stylurus olivaceus) in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Paul Catling, Co-chair of the COSEWIC Arthropods Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur le gomphe olive (Stylurus olivaceus) au Canada. Cover illustration/photo: Olive Clubtail — Photo by Jim Johnson. Permission granted for reproduction. ©Her Majesty the Queen in Right of Canada, 2011. Catalogue No. CW69-14/637-2011E-PDF ISBN 978-1-100-18707-5 Recycled paper COSEWIC Assessment Summary Assessment Summary – May 2011 Common name Olive Clubtail Scientific name Stylurus olivaceus Status Endangered Reason for designation This highly rare, stream-dwelling dragonfly with striking blue eyes is known from only 5 locations within three separate regions of British Columbia. -

Boreal Snaketail
Species Status Assessment Class: Insecta Family: Gomphidae Scientific Name: Ophiogomphus colubrinus Common Name: Boreal snaketail Species synopsis: As its name implies, the boreal snaketail (Ophiogomphus colubrinus) is a species of northern distribution, and it has the most northern range of any clubtail (Mead 2003). The range extends from the western provinces of British Columbia and Alberta, eastward across Canada, to Ontario, Quebec, and New Brunswick. In the United States, it occurs in Maine, New Hampshire, and New York, as well as in Michigan, Wisconsin, Minnesota, and Wyoming (Needham et al. 2000). O. colubrinus was first documented in New York in 1995, with a number of subsequent records in 1996. All of these records are from the Ausable River in the central Adirondacks, including both the East and West Branch. Some of the recorded locations were documented only by the collection of exuviae. Although the original New York location, the Ausable River along Riverside Drive near Lake Placid, and nearby stretches of the Ausable were searched on several occasions, presence was not documented during the New York State Dragonfly and Damselfly Survey (NYDDS). There is no evidence that changes have occurred in the Ausable River in the vicinity of the previously documented records, so additional surveys would be desirable to confirm the continued presence of this species in New York (White et al. 2010). Previously recorded locations for O. colubrinus in New York are on rivers, principally nearer to the headwaters where the rivers are rapid and shallow with sand, gravel, rock, and boulder substrate, and are primarily bordered by trees and shrubs (New York Natural Heritage Program 2010). -

Ecography ECOG-02578 Pinkert, S., Brandl, R
Ecography ECOG-02578 Pinkert, S., Brandl, R. and Zeuss, D. 2016. Colour lightness of dragonfly assemblages across North America and Europe. – Ecography doi: 10.1111/ecog.02578 Supplementary material Appendix 1 Figures A1–A12, Table A1 and A2 1 Figure A1. Scatterplots between female and male colour lightness of 44 North American (Needham et al. 2000) and 19 European (Askew 1988) dragonfly species. Note that colour lightness of females and males is highly correlated. 2 Figure A2. Correlation of the average colour lightness of European dragonfly species illustrated in both Askew (1988) and Dijkstra and Lewington (2006). Average colour lightness ranges from 0 (absolute black) to 255 (pure white). Note that the extracted colour values of dorsal dragonfly drawings from both sources are highly correlated. 3 Figure A3. Frequency distribution of the average colour lightness of 152 North American and 74 European dragonfly species. Average colour lightness ranges from 0 (absolute black) to 255 (pure white). Rugs at the abscissa indicate the value of each species. Note that colour values are from different sources (North America: Needham et al. 2000, Europe: Askew 1988), and hence absolute values are not directly comparable. 4 Figure A4. Scatterplots of single ordinary least-squares regressions between average colour lightness of 8,127 North American dragonfly assemblages and mean temperature of the warmest quarter. Red dots represent assemblages that were excluded from the analysis because they contained less than five species. Note that those assemblages that were excluded scatter more than those with more than five species (c.f. the coefficients of determination) due to the inherent effect of very low sampling sizes. -

Introduction to Odonata: Dragonflies & Damselflies
Introduction to Odonata: Dragonflies & Damselflies Ornate Pennant (Celithemis ornata), C. Mazzacano What are odonates? “toothed” fossil record >400 million years Proto-fossils from Permian with 27 in. (68 cm) wingspan Protolindenia wittei fossil; 155 million y.o.; www.ucmp.berkeley.edu/arthropoda/uniramia/odonatoida.html What are odonates? Diverse; 5952 species globally 463 species in North America (Schorr & Paulson, 2013) Blue Dasher (Pachydiplax longipennis), C. Mazzacano Anisoptera (dragonflies) Zygoptera (damselflies) Sierra Madre Dancer (Argia lacrimans), C. Mazzacano What are odonates? Colorful Double-striped Bluet (Enallagma basidens), C. Mazzacano Ebony Jewelwing, (Calopteryx maculata) John Wallace What are odonates? Aquatic Alexa Carleton Celeste Mazzacano What are odonates? Hunters… Dennis Paulson …and prey Larry Rea What are odonates? Symbolic Gila National Forest; USFS Alexa Carleton Petroglyph National Monument What are odonates? American Rubyspot (Hetaerina cruentata), C. Mazzacano Variable Darner (Aeshna interrupta), C. Mazzacano Beautiful! Eastern Amberwing (Perithemis tenera), John Abbott Carmine Skimmer (Orthemis discolor), C. Mazzacano Dragonfly or damselfly? Common Green Darner (Anax junius), Dennis Paulson Powdered Dancer (Argia moesta), C. Mazzacano Large body Smaller body Wider abdomen Slender abdomen Eyes touch or nearly so Eyes separated Hindwings broader than Equal-sized wings forewings stalked at base Wings held horizontal Wings folded above or along when perched body when perched Odonate habitats Bear Creek CA; C. Mazzacano Cache la Poudre River CO; C. Mazzacano Running water: rivers, streams, creeks, ditches Whychus Creek OR; C. Mazzacano Sandy River OR; C. Mazzacano Odonate habitats Deweys Mill Pond, Quechee VT; C. Mazzacano RAMSAR wetland, Xalapa Mexico; C. Mazzacano Still water: marsh, swamp, bog, seep, wet meadow, lake, pond Kristi Lake bog, Saskatchewan Canada; C. -

A Checklist of North American Odonata
A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution Dennis R. Paulson and Sidney W. Dunkle 2009 Edition (updated 14 April 2009) A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution 2009 Edition (updated 14 April 2009) Dennis R. Paulson1 and Sidney W. Dunkle2 Originally published as Occasional Paper No. 56, Slater Museum of Natural History, University of Puget Sound, June 1999; completely revised March 2009. Copyright © 2009 Dennis R. Paulson and Sidney W. Dunkle 2009 edition published by Jim Johnson Cover photo: Tramea carolina (Carolina Saddlebags), Cabin Lake, Aiken Co., South Carolina, 13 May 2008, Dennis Paulson. 1 1724 NE 98 Street, Seattle, WA 98115 2 8030 Lakeside Parkway, Apt. 8208, Tucson, AZ 85730 ABSTRACT The checklist includes all 457 species of North American Odonata considered valid at this time. For each species the original citation, English name, type locality, etymology of both scientific and English names, and approxi- mate distribution are given. Literature citations for original descriptions of all species are given in the appended list of references. INTRODUCTION Before the first edition of this checklist there was no re- Table 1. The families of North American Odonata, cent checklist of North American Odonata. Muttkows- with number of species. ki (1910) and Needham and Heywood (1929) are long out of date. The Zygoptera and Anisoptera were cov- Family Genera Species ered by Westfall and May (2006) and Needham, West- fall, and May (2000), respectively, but some changes Calopterygidae 2 8 in nomenclature have been made subsequently. Davies Lestidae 2 19 and Tobin (1984, 1985) listed the world odonate fauna Coenagrionidae 15 103 but did not include type localities or details of distri- Platystictidae 1 1 bution. -

Pygmy Snaketail, Ophiogomphus Howei, Prepared Under Contract with Environment and Climate Change Canada
COSEWIC Assessment and Status Report on the Pygmy Snaketail Ophiogomphus howei in Canada SPECIAL CONCERN 2018 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2018. COSEWIC assessment and status report on the Pygmy Snaketail Ophiogomphus howei in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 46 pp. (http://www.registrelep-sararegistry.gc.ca/default.asp?lang=en&n=24F7211B-1). Previous report(s): COSEWIC. 2008. COSEWIC assessment and status report on the Pygmy Snaketail Ophiogomphus howei in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 34 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC would like to acknowledge John Klymko for writing the status report on Pygmy Snaketail, Ophiogomphus howei, prepared under contract with Environment and Climate Change Canada. This report was overseen and edited by Paul Grant, COSEWIC Arthropods Specialist Subcommittee Co-chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment and Climate Change Canada Ottawa, ON K1A 0H3 Tel.: 819-938-4125 Fax: 819-938-3984 E-mail: [email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur L’ophiogomphe de Howe (Ophiogomphus howei) au Canada. Cover illustration/photo: Pygmy Snaketail — Cover photo by: Denis Doucet. Her Majesty the Queen in Right of Canada, 2018. Catalogue No. CW69-14/542-2019E-PDF ISBN 978-0-660-31321-4 COSEWIC Assessment Summary Assessment Summary – November 2018 Common name Pygmy Snaketail Scientific name Ophiogomphus howei Status Special Concern Reason for designation One of Canada’s smallest dragonflies, this globally-rare species is a habitat specialist, restricted to a few rivers in New Brunswick and a single river in northwestern Ontario. -

A Checklist of North American Odonata, 2021 1 Each Species Entry in the Checklist Is a Paragraph In- Table 2
A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution Dennis R. Paulson and Sidney W. Dunkle 2021 Edition (updated 12 February 2021) A Checklist of North American Odonata Including English Name, Etymology, Type Locality, and Distribution 2021 Edition (updated 12 February 2021) Dennis R. Paulson1 and Sidney W. Dunkle2 Originally published as Occasional Paper No. 56, Slater Museum of Natural History, University of Puget Sound, June 1999; completely revised March 2009; updated February 2011, February 2012, October 2016, November 2018, and February 2021. Copyright © 2021 Dennis R. Paulson and Sidney W. Dunkle 2009, 2011, 2012, 2016, 2018, and 2021 editions published by Jim Johnson Cover photo: Male Calopteryx aequabilis, River Jewelwing, from Crab Creek, Grant County, Washington, 27 May 2020. Photo by Netta Smith. 1 1724 NE 98th Street, Seattle, WA 98115 2 8030 Lakeside Parkway, Apt. 8208, Tucson, AZ 85730 ABSTRACT The checklist includes all 471 species of North American Odonata (Canada and the continental United States) considered valid at this time. For each species the original citation, English name, type locality, etymology of both scientific and English names, and approximate distribution are given. Literature citations for original descriptions of all species are given in the appended list of references. INTRODUCTION We publish this as the most comprehensive checklist Table 1. The families of North American Odonata, of all of the North American Odonata. Muttkowski with number of species. (1910) and Needham and Heywood (1929) are long out of date. The Anisoptera and Zygoptera were cov- Family Genera Species ered by Needham, Westfall, and May (2014) and West- fall and May (2006), respectively. -
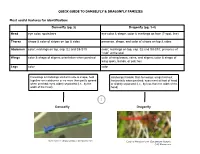
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

Agrion 22(2) - July 2018 AGRION NEWSLETTER of the WORLDWIDE DRAGONFLY ASSOCIATION
Agrion 22(2) - July 2018 AGRION NEWSLETTER OF THE WORLDWIDE DRAGONFLY ASSOCIATION PATRON: Professor Edward O. Wilson FRS, FRSE Volume 22, Number 2 July 2018 Secretary: Dr. Jessica I. Ware, Assistant Professor, Department of Biological Sciences, 206 Boyden Hall, Rutgers University, 195 University Avenue, Newark, NJ 07102, USA. Email: [email protected]. Editors: Keith D.P. Wilson. 18 Chatsworth Road, Brighton, BN1 5DB, UK. Email: [email protected]. Graham T. Reels. 31 St Anne’s Close, Badger Farm, Winchester, SO22 4LQ, Hants, UK. Email: [email protected]. ISSN 1476-2552 Agrion 22(2) - July 2018 AGRION NEWSLETTER OF THE WORLDWIDE DRAGONFLY ASSOCIATION AGRION is the Worldwide Dragonfly Association’s (WDA’s) newsletter, published twice a year, in January and July. The WDA aims to advance public education and awareness by the promotion of the study and conservation of dragonflies (Odonata) and their natural habitats in all parts of the world. AGRION covers all aspects of WDA’s activities; it communicates facts and knowledge related to the study and conservation of dragonflies and is a forum for news and information exchange for members. AGRION is freely available for downloading from the WDA website at [https://worlddragonfly.org/publications/]. WDA is a Registered Charity (Not-for-Profit Organization), Charity No. 1066039/0. A ‘pdf’ of the WDA’s Constitutionand and byelaws can be found at its website link at [https://worlddragonfly. org/wda/] ________________________________________________________________________________ Editor’s notes Keith Wilson [[email protected]] WDA Membership There are several kinds of WDA membership available either with or without the WDA’s journal (The International Journal of Odonatology). -

Prioritizing Odonata for Conservation Action in the Northeastern USA
APPLIED ODONATOLOGY Prioritizing Odonata for conservation action in the northeastern USA Erin L. White1,4, Pamela D. Hunt2,5, Matthew D. Schlesinger1,6, Jeffrey D. Corser1,7, and Phillip G. deMaynadier3,8 1New York Natural Heritage Program, State University of New York College of Environmental Science and Forestry, 625 Broadway 5th Floor, Albany, New York 12233-4757 USA 2Audubon Society of New Hampshire, 84 Silk Farm Road, Concord, New Hampshire 03301 USA 3Maine Department of Inland Fisheries and Wildlife, 650 State Street, Bangor, Maine 04401 USA Abstract: Odonata are valuable biological indicators of freshwater ecosystem integrity and climate change, and the northeastern USA (Virginia to Maine) is a hotspot of odonate diversity and a region of historical and grow- ing threats to freshwater ecosystems. This duality highlights the urgency of developing a comprehensive conser- vation assessment of the region’s 228 resident odonate species. We offer a prioritization framework modified from NatureServe’s method for assessing conservation status ranks by assigning a single regional vulnerability metric (R-rank) reflecting each species’ degree of relative extinction risk in the northeastern USA. We calculated the R-rank based on 3 rarity factors (range extent, area of occupancy, and habitat specificity), 1 threat factor (vulnerability of occupied habitats), and 1 trend factor (relative change in range size). We combine this R-rank with the degree of endemicity (% of the species’ USA and Canadian range that falls within the region) as a proxy for regional responsibility, thereby deriving a list of species of combined vulnerability and regional management responsibility. Overall, 18% of the region’s odonate fauna is imperiled (R1 and R2), and peatlands, low-gradient streams and seeps, high-gradient headwaters, and larger rivers that harbor a disproportionate number of these species should be considered as priority habitat types for conservation. -

Recovery Strategy for the Pygmy Snaketail in Ontario
Photo: Denis Doucet Pygmy Snaketail (Ophiogomphus howei) in Ontario Ontario Recovery Strategy Series Recovery strategy prepared under the Endangered Species Act, 2007 Ministry of Natural Resources About the Ontario Recovery Strategy Series This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada. What is recovery? What’s next? Recovery of species at risk is the process by which the Nine months after the completion of a recovery strategy decline of an endangered, threatened, or extirpated a government response statement will be published species is arrested or reversed, and threats are which summarizes the actions that the Government of removed or reduced to improve the likelihood of a Ontario intends to take in response to the strategy. species’ persistence in the wild. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and What is a recovery strategy? conservationists. Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to For more information achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the To learn more about species at risk recovery in Ontario, survival and recovery of the species. It also makes please visit the Ministry of Natural Resources Species at recommendations on the objectives for protection and Risk webpage at: www.ontario.ca/speciesatrisk recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. -

Odonata: Gomphidae) in a Northern Wisconsin River
International Journal of Odonatology, 2016 Vol. 19, Nos. 1–2, 83–93, http://dx.doi.org/10.1080/13887890.2016.1183525 Pre-emergent movements and survival of F-0 larvae of Ophiogomphus rupinsulensis (Odonata: Gomphidae) in a northern Wisconsin river Robert B. DuBoisa∗ and William A. Smithb aDepartment of Natural Resources, Bureau of Natural Heritage Conservation, Superior, WI, USA bDepartment of Natural Resources, Bureau of Natural Heritage Conservation, Madison, WI, USA (Received 11 March 2016; final version received 25 April 2016) We marked and released 276 F-0 larvae of Ophiogomphus rupinsulensis in the fall of 2008 in a 99-m riffle (marking riffle) of a small, serially discontinuous, northern Wisconsin river (USA). We then recovered marked exuviae via exhaustive collecting on the banks of a 272-m sampling reach in which the marking riffle was located during spring of 2009. We collected 6054 exuviae of O. rupinsulensis along the sampling reach, including 3829 exuviae along the marking riffle (19.3 exuviae m–1). Mark retention was complete and our ability to recover marked exuviae in the field was high (92%). We recovered 38 marked exuviae which provided a minimum estimate of survival (14%) for F-0 larvae from late September to the following June. The density of F-0 larvae in the marking riffle in late September was estimated at 22.6 larvae m–2. Nearly all F-0 larvae made small downstream movements (97% moved < 60 m) at some time during the 36 weeks before emergence, but they did not make substantial longitudinal movements. These results affirmed the premise that locations of found exuviae of O.