The Case of Deirocheline Turtles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predators As Agents of Selection and Diversification
diversity Review Predators as Agents of Selection and Diversification Jerald B. Johnson * and Mark C. Belk Evolutionary Ecology Laboratories, Department of Biology, Brigham Young University, Provo, UT 84602, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-801-422-4502 Received: 6 October 2020; Accepted: 29 October 2020; Published: 31 October 2020 Abstract: Predation is ubiquitous in nature and can be an important component of both ecological and evolutionary interactions. One of the most striking features of predators is how often they cause evolutionary diversification in natural systems. Here, we review several ways that this can occur, exploring empirical evidence and suggesting promising areas for future work. We also introduce several papers recently accepted in Diversity that demonstrate just how important and varied predation can be as an agent of natural selection. We conclude that there is still much to be done in this field, especially in areas where multiple predator species prey upon common prey, in certain taxonomic groups where we still know very little, and in an overall effort to actually quantify mortality rates and the strength of natural selection in the wild. Keywords: adaptation; mortality rates; natural selection; predation; prey 1. Introduction In the history of life, a key evolutionary innovation was the ability of some organisms to acquire energy and nutrients by killing and consuming other organisms [1–3]. This phenomenon of predation has evolved independently, multiple times across all known major lineages of life, both extinct and extant [1,2,4]. Quite simply, predators are ubiquitous agents of natural selection. Not surprisingly, prey species have evolved a variety of traits to avoid predation, including traits to avoid detection [4–6], to escape from predators [4,7], to withstand harm from attack [4], to deter predators [4,8], and to confuse or deceive predators [4,8]. -
Skin Patterning in Octopus Vulgaris and Its Importance for Camouflage
Skin patterning in Octopus vulgaris anditsimportance for camouflage DORSAL ANTERiOR POSTERIOR VENTRAL Wilma Meijer-Kuiper D490 Dqo "Scriptie"entitled: j Skin patterning in Octopus vulgaris and its importance for camouflage Author: Wilma Meijer-Kuiper. Supervisor: Dr. P.J. Geerlink. April, 1993. Cover figure from Wells, 1978. Department of Marine Biology, University of Groningen, Kerklaan 30, 9750 AA Haren, The Netherlands. TABLE OF CONTENTS PAGE Abstract 1 1. Introduction 2 1.1. Camouflage in marine invertebrates 2 1 .2. Cephalopods 2 2. General biology of the octopods 4 2.1. Octopus vulgar/s 4 3. Skin patterning in Octopus 6 3.1. Patterns 6 3.2. Components 8 3.3. Units 9 3.4. Elements 10 3.4.1. Chromatophores 10 3.4.2. Reflector cells and iridophores 16 3.4.3. Leucophores 18 4. The chromatic unit 20 5. Nervous system 22 5.1. Motor units 22 5.2. Electrical stimulation 22 5.3. Projection of nerves on the skin surface 24 5.4. The nervous control system 25 5.5. Neurotransmitters 27 6. Vision in cephalopods, particularly in O.vu!garis 28 6.1. Electro-physiological experiments 28 6.2. Photopigments and receptor cells 28 6.3. Behavioural (training) experiments 29 6.4. Eye movements and optomotor responses 29 7. Camouflage in a colour-blind animal 31 7.1. Matching methods 31 7.2. Countershading reflex 35 8. Conclusive remarks 36 Literature 38 ABSTRACT Camouflage is a method by which animals obtain concealment from other animals by blending in with their environment. Of the cephalopods, the octopods have an extra-ordinary ability to match their surroundings by changing the colour and texture of their skin. -

N REPTILIA: SQUAMATA: SAURIA: PHRYNOSOMATIDAE PHRYNOSOMA Phrynosoma Modestum Girard
630.1 n REPTILIA: SQUAMATA: SAURIA: PHRYNOSOMATIDAE PHRYNOSOMAMODESTUM Catalogue of American Amphibians and Reptiles. Whiting, M.J. and J.R. Dixon. 1996. Phrynosoma modestum. Phrynosoma modestum Girard Roundtail Homed Lizard Phrynosoma modesturn Girard, in Baird and Girard, 1852:69 (see Banta, 1971). Type-locality, "from the valley of the Rio Grande west of San Antonio .....and from between San Antonio and El Paso del Norte." Syntypes, National Mu- seum of Natural History (USNM) 164 (7 specimens), sub- Figure. Adult Phrynosoma modestum from Doha Ana County, adult male, adult male, and 5 adult females, USNM 165660, New Mexico. Photograph by Suzanne L. Collins, courtesy of an adult male, and Museum of Natural History, University The Center for North American Amphibians and Reptiles. of Illinois at Urbana-Champaign (UIMNH) 40746, an adult male, collected by J.H. lark in May or June 1851 (Axtell, 1988) (not examined by authors). See Remarks. Phrynosomaplatyrhynus: Hemck,Terry, and Hemck, 1899: 136. Doliosaurus modestus: Girard, 1858:409. Phrynosoma modestrum: Morafka, Adest, Reyes, Aguirre L., A(nota). modesta: Cope, 1896:834. and Lieberman, 1992:2 14. Lapsus. Content. No subspecies have been described. and Degenhardt et al. (1996). Habitat photographs appeared in Sherbrooke (1981) and Switak (1979). Definition. Phrynosoma modestum is the smallest horned liz- ard, with a maximum SVL of 66 mm in males and 71 mm in Distribution. Phrynosoma modestum occurs in southern and females (Fitch, 1981). It is the sister taxon to l? platyrhinos, western Texas, southern New Mexico, southeastern Arizona and and is part of the "northern radiation" (sensu Montanucci, 1987). north-central Mexico. -
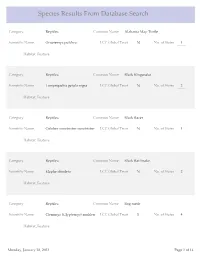
Species Results from Database Search
Species Results From Database Search Category Reptiles Common Name Alabama Map Turtle Scientific Name Graptemys pulchra LCC Global Trust N No. of States 1 Habitat_Feature Category Reptiles Common Name Black Kingsnake Scientific Name Lampropeltis getula nigra LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Black Racer Scientific Name Coluber constrictor constrictor LCC Global Trust N No. of States 1 Habitat_Feature Category Reptiles Common Name Black Rat Snake Scientific Name Elaphe obsoleta LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Bog turtle Scientific Name Clemmys (Glyptemys) muhlen LCC Global Trust Y No. of States 4 Habitat_Feature Monday, January 28, 2013 Page 1 of 14 Category Reptiles Common Name Broadhead Skink Scientific Name Eumeces laticeps LCC Global Trust N No. of States 5 Habitat_Feature Category Reptiles Common Name Coal Skink Scientific Name Eumeces anthracinus LCC Global Trust Y No. of States 8 Habitat_Feature Category Reptiles Common Name Common Five-lined Skink Scientific Name Eumeces fasciatus LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Common Map Turtle Scientific Name Graptemys geographica LCC Global Trust N No. of States 6 Habitat_Feature Category Reptiles Common Name Common Musk Turtle Scientific Name Sternotherus odoratus LCC Global Trust N No. of States 2 Habitat_Feature Monday, January 28, 2013 Page 2 of 14 Category Reptiles Common Name Common Ribbonsnake Scientific Name Thamnophis sauritus sauritus LCC Global Trust N No. of States 6 Habitat_Feature Category Reptiles Common Name Common Snapping Turtle Scientific Name Chelydra serpentina LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Corn snake Scientific Name Elaphe guttata guttata LCC Global Trust N No. -
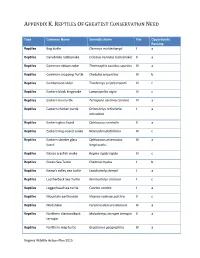
Reptiles of Greatest Conservation Need
APPENDIX K. REPTILES OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Reptiles Bog turtle Clemmys muhlenbergii I a Reptiles Canebrake rattlesnake Crotalus horridus (canebrake) II a Reptiles Common ribbonsnake Thamnophis sauritus sauritus IV a Reptiles Common snapping Turtle Chelydra serpentina IV b Reptiles Cumberland slider Trachemys scripta troostii III c Reptiles Eastern black kingsnake Lampropeltis nigra III c Reptiles Eastern box turtle Terrapene carolina carolina III a Reptiles Eastern chicken turtle Deirochelys reticularia I a reticularia Reptiles Eastern glass lizard Ophisaurus ventralis II a Reptiles Eastern hog-nosed snake Heterodon platirhinos IV c Reptiles Eastern slender glass Ophisaurus attenuatus IV a lizard longicaudus Reptiles Glossy crayfish snake Regina rigida rigida III c Reptiles Green Sea Turtle Chelonia mydas I b Reptiles Kemp's ridley sea turtle Lepidochelys kempii I a Reptiles Leatherback Sea Turtle Dermochelys coriacea I c Reptiles Loggerhead sea turtle Caretta caretta I a Reptiles Mountain earthsnake Virginia valeriae pulchra II c Reptiles Mudsnake Farancia abacura abacura IV a Reptiles Northern diamondback Malaclemys terrapin terrapin II a terrapin Reptiles Northern map turtle Graptemys geographica IV a Virginia Wildlife Action Plan 2015 APPENDIX K. REPTILES OF GREATEST CONSERVATION NEED Reptiles Northern pinesnake Pituophis melanoleucus I a melanoleucus Reptiles Queen snake Regina septemvittata IV a Reptiles Rainbow snake Farancia erytrogramma IV a erytrogramma Reptiles -

Il/I,E,Icanjluseum
il/i,e,icanJluseum PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK 24, N.Y. NUMBER 1870 FEBRUARY 26, 1958 The Role of the "Third Eye" in Reptilian Behavior BY ROBERT C. STEBBINS1 AND RICHARD M. EAKIN2 INTRODUCTION The pineal gland remains an organ of uncertain function despite extensive research (see summaries of literature: Pflugfelder, 1957; Kitay and Altschule, 1954; and Engel and Bergmann, 1952). Its study by means of pinealectomy has been hampered in the higher vertebrates by its recessed location and association with large blood vessels which have made difficult its removal without brain injury or serious hemorrhage. Lack of purified, standardized extracts, improper or inadequate extrac- tion techniques (Quay, 1956b), and lack of suitable assay methods to test biological activity have hindered the physiological approach. It seems probable that the activity of the gland varies among different species (Engel and Bergmann, 1952), between individuals of the same species, and within the same individual. This may also have contrib- uted to the variable results obtained with pinealectomy, injection, and implantation experiments. The morphology of the pineal apparatus is discussed in detail by Tilney and Warren (1919) and Gladstone and Wakely (1940). Only a brief survey is presented here for orientation. In living vertebrates the pineal system in its most complete form may be regarded as consisting of a series of outgrowths situated above the third ventricle in the roof of the diencephalon. In sequence these outgrowths are the paraphysis, dorsal sac, parapineal, and pineal bodies. The paraphysis, the most I University of California Museum of Vertebrate Zoology. -

The Biochromes ) 1.2
FORSCHUNG 45 CHIMIA 49 (1995) Nr. 3 (Miirz) Chim;a 49 (1995) 45-68 quire specific molecules, pigments or dyes © Neue Schweizerische Chemische Gesellschaft (biochromes) or systems containing them, /SSN 0009-4293 to absorb the light energy. Photoprocesses and colors are essential for life on earth, and without these biochromes and the photophysical and photochemical interac- tions, life as we know it would not have The Function of Natural been possible [1][2]. a Colorants: The Biochromes ) 1.2. Notation The terms colorants, dyes, and pig- ments ought to be used in the following way [3]: Colorants are either dyes or pig- Hans-Dieter Martin* ments, the latter being practically insolu- ble in the media in which they are applied. Indiscriminate use of these terms is fre- Abstract. The colors of nature belong undoubtedly to the beautiful part of our quently to be found in literature, but in environment. Colors always fascinated humans and left them wonderstruck. But the many biological systems it is not possible trivial question as to the practical application of natural colorants led soon and at all to make this differentiation. The consequently to coloring and dyeing of objects and humans. Aesthetical, ritual and coloring compounds of organisms have similar aspects prevailed. This function of dyes and pigments is widespread in natl)re. been referred to as biochromes, and this The importance of such visual-effective dyes is obvious: they support communication seems to be a suitable expression for a between organisms with the aid of conspicuous optical signals and they conceal biological colorant, since it circumvents revealing ones, wl,1eninconspicuosness can mean survival. -

In AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): Species in Red = Depleted to the Point They May Warrant Federal Endangered Species Act Listing
Southern and Midwestern Turtle Species Affected by Commercial Harvest (in AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): species in red = depleted to the point they may warrant federal Endangered Species Act listing Common snapping turtle (Chelydra serpentina) – AR, GA, IA, KY, MO, OH, OK, SC, TX Florida common snapping turtle (Chelydra serpentina osceola) - FL Southern painted turtle (Chrysemys dorsalis) – AR Western painted turtle (Chrysemys picta) – IA, MO, OH, OK Spotted turtle (Clemmys gutatta) - FL, GA, OH Florida chicken turtle (Deirochelys reticularia chrysea) – FL Western chicken turtle (Deirochelys reticularia miaria) – AR, FL, GA, KY, MO, OK, TN, TX Barbour’s map turtle (Graptemys barbouri) - FL, GA Cagle’s map turtle (Graptemys caglei) - TX Escambia map turtle (Graptemys ernsti) – FL Common map turtle (Graptemys geographica) – AR, GA, OH, OK Ouachita map turtle (Graptemys ouachitensis) – AR, GA, OH, OK, TX Sabine map turtle (Graptemys ouachitensis sabinensis) – TX False map turtle (Graptemys pseudogeographica) – MO, OK, TX Mississippi map turtle (Graptemys pseuogeographica kohnii) – AR, TX Alabama map turtle (Graptemys pulchra) – GA Texas map turtle (Graptemys versa) - TX Striped mud turtle (Kinosternon baurii) – FL, GA, SC Yellow mud turtle (Kinosternon flavescens) – OK, TX Common mud turtle (Kinosternon subrubrum) – AR, FL, GA, OK, TX Alligator snapping turtle (Macrochelys temminckii) – AR, FL, GA, LA, MO, TX Diamond-back terrapin (Malaclemys terrapin) – FL, GA, LA, SC, TX River cooter (Pseudemys concinna) – AR, FL, -

Pigment Pattern Formation in Zebrafish 443
MICROSCOPY RESEARCH AND TECHNIQUE 58:442–455 (2002) Pigment Pattern Formation in Zebrafish: A Model for Developmental Genetics and the Evolution of Form 1 1,2 IAN K. QUIGLEY AND DAVID M. PARICHY * 1Section of Integrative Biology, University of Texas at Austin, Austin, Texas 78712 2Section of Molecular, Cell and Developmental Biology and Institute for Cellular and Molecular Biology University of Texas at Austin, Austin, Texas 78712 ABSTRACT The zebrafish Danio rerio is an emerging model organism for understanding vertebrate development and genetics. One trait of both historical and recent interest is the pattern formed by neural crest–derived pigment cells, or chromatophores, which include black melano- phores, yellow xanthophores, and iridescent iridophores. In zebrafish, an embryonic and early larval pigment pattern consists of several stripes of melanophores and iridophores, whereas xanthophores are scattered widely over the flank. During metamorphosis, however, this pattern is transformed into that of the adult, which comprises several dark stripes of melanophores and iridophores that alternate with light stripes of xanthophores and iridophores. In this review, we place zebrafish relative to other model and non-model species; we review what is known about the processes of chromatophore specification, differentiation, and morphogenesis during the develop- ment of embryonic and adult pigment patterns, and we address how future studies of zebrafish will likely aid our understanding of human disease and the evolution of form. Microsc. Res. Tech. 58: 442–455, 2002. © 2002 Wiley-Liss, Inc. INTRODUCTION number of genetic mutants have been isolated that To generate the adult body plan, cells must differen- affect pigmentation. For example, a variety of pigment tiate, proliferate, die, and migrate. -

Does Mutual Sexual Selection Explain the Evolution of Head Crests in Pterosaurs and Dinosaurs?
LETHAIA REVIEW Does mutual sexual selection explain the evolution of head crests in pterosaurs and dinosaurs? DAVID W.E. HONE, DARREN NAISH AND INNES C. CUTHILL Hone, D.W.E., Naish, D. & Cuthill, I.C. 2011: Does mutual sexual selection explain the evolution of head crests in pterosaurs and dinosaurs? Lethaia, DOI: 10.1111/j.1502- 3931.2011.00300.x Cranial ornamentation is widespread throughout the extinct non-avialian Ornithodira, being present throughout Pterosauria, Ornithischia and Saurischia. Ornaments take many forms, and can be composed of at least a dozen different skull bones, indicating multiple origins. Many of these crests serve no clear survival function and it has been suggested that their primary use was for species recognition or sexual display. The distribution within Ornithodira and the form and position of these crests suggest sexual selection as a key factor, although the role of the latter has often been rejected on the grounds of an apparent lack of sexual dimorphism in many species. Surprisingly, the phenomenon of mutual sexual selection – where both males and females are ornamented and both select mates – has been ignored in research on fossil ornithodirans, despite a rich history of research and frequent expression in modern birds. Here, we review the available evidence for the functions of ornithodiran cranial crests and conclude that mutual sexual selection presents a valid hypothesis for their presence and distribution. The integration of mutual sexual selection into future studies is critical to our under- standing of ornithodiran ecology, evolution and particularly questions regarding sexual dimorphism. h Behaviour, Dinosauria, ornaments, Pterosauria, sexual selection. -

The Biology and Ecology of the Common Cuttlefish (Sepia Officinalis)
Supporting Sustainable Sepia Stocks Report 1: The biology and ecology of the common cuttlefish (Sepia officinalis) Daniel Davies Kathryn Nelson Sussex IFCA 2018 Contents Summary ................................................................................................................................................. 2 Acknowledgements ................................................................................................................................. 2 Introduction ............................................................................................................................................ 3 Biology ..................................................................................................................................................... 3 Physical description ............................................................................................................................ 3 Locomotion and respiration ................................................................................................................ 4 Vision ................................................................................................................................................... 4 Chromatophores ................................................................................................................................. 5 Colour patterns ................................................................................................................................... 5 Ink sac and funnel organ -

Invasion of the Turtles? Wageningen Approach
Alterra is part of the international expertise organisation Wageningen UR (University & Research centre). Our mission is ‘To explore the potential of nature to improve the quality of life’. Within Wageningen UR, nine research institutes – both specialised and applied – have joined forces with Wageningen University and Van Hall Larenstein University of Applied Sciences to help answer the most important questions in the domain of healthy food and living environment. With approximately 40 locations (in the Netherlands, Brazil and China), 6,500 members of staff and 10,000 students, Wageningen UR is one of the leading organisations in its domain worldwide. The integral approach to problems and the cooperation between the exact sciences and the technological and social disciplines are at the heart of the Invasion of the turtles? Wageningen Approach. Alterra is the research institute for our green living environment. We offer a combination of practical and scientific Exotic turtles in the Netherlands: a risk assessment research in a multitude of disciplines related to the green world around us and the sustainable use of our living environment, such as flora and fauna, soil, water, the environment, geo-information and remote sensing, landscape and spatial planning, man and society. Alterra report 2186 ISSN 1566-7197 More information: www.alterra.wur.nl/uk R.J.F. Bugter, F.G.W.A. Ottburg, I. Roessink, H.A.H. Jansman, E.A. van der Grift and A.J. Griffioen Invasion of the turtles? Commissioned by the Invasive Alien Species Team of the Food and Consumer Product Safety Authority Invasion of the turtles? Exotic turtles in the Netherlands: a risk assessment R.J.F.