Invasion of the Turtles? Wageningen Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

13914444D46c0aa91d02e31218
2 Breeding of wild and some domestic animals at regional zoological institutions in 2013 3 РЫБЫ P I S C E S ВОББЕЛОНГООБРАЗНЫЕ ORECTOLOBIFORMES Сем. Азиатские кошачьи акулы (Бамбуковые акулы) – Hemiscyllidae Коричневополосая бамбуковая акула – Chiloscyllium punctatum Brownbanded bambooshark IUCN (NT) Sevastopol 20 ХВОСТОКОЛООБРАЗНЫЕ DASYATIFORMES Сем. Речные хвостоколы – Potamotrygonidae Глазчатый хвостокол (Моторо) – Potamotrygon motoro IUCN (DD) Ocellate river stingray Sevastopol - ? КАРПООБРАЗНЫЕ CYPRINIFORMES Сем. Цитариновые – Citharinidae Серебристый дистиход – Distichodusaffinis (noboli) Silver distichodus Novosibirsk 40 Сем. Пираньевые – Serrasalmidae Серебристый метиннис – Metynnis argenteus Silver dollar Yaroslavl 10 Обыкновенный метиннис – Metynnis schreitmuelleri (hypsauchen) Plainsilver dollar Nikolaev 4; Novosibirsk 100; Kharkov 20 Пятнистый метиннис – Metynnis maculatus Spotted metynnis Novosibirsk 50 Пиранья Наттерера – Serrasalmus nattereri Red piranha Novosibirsk 80; Kharkov 30 4 Сем. Харацидовые – Characidae Красноплавничный афиохаракс – Aphyocharax anisitsi (rubripinnis) Bloodfin tetra Киев 5; Perm 10 Парагвайский афиохаракс – Aphyocharax paraquayensis Whitespot tetra Perm 11 Рубиновый афиохаракс Рэтбина – Aphyocharax rathbuni Redflank bloodfin Perm 10 Эквадорская тетра – Astyanax sp. Tetra Perm 17 Слепая рыбка – Astyanax fasciatus mexicanus (Anoptichthys jordani) Mexican tetra Kharkov 10 Рублик-монетка – Ctenobrycon spilurus (+ С. spilurusvar. albino) Silver tetra Kharkov 20 Тернеция (Траурная тетра) – Gymnocorymbus -

Competing Generic Concepts for Blanding's, Pacific and European
Zootaxa 2791: 41–53 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) Competing generic concepts for Blanding’s, Pacific and European pond turtles (Emydoidea, Actinemys and Emys)—Which is best? UWE FRITZ1,3, CHRISTIAN SCHMIDT1 & CARL H. ERNST2 1Museum of Zoology, Senckenberg Dresden, A. B. Meyer Building, D-01109 Dresden, Germany 2Division of Amphibians and Reptiles, MRC 162, Smithsonian Institution, P.O. Box 37012, Washington, D.C. 20013-7012, USA 3Corresponding author. E-mail: [email protected] Abstract We review competing taxonomic classifications and hypotheses for the phylogeny of emydine turtles. The formerly rec- ognized genus Clemmys sensu lato clearly is paraphyletic. Two of its former species, now Glyptemys insculpta and G. muhlenbergii, constitute a well-supported basal clade within the Emydinae. However, the phylogenetic position of the oth- er two species traditionally placed in Clemmys remains controversial. Mitochondrial data suggest a clade embracing Actinemys (formerly Clemmys) marmorata, Emydoidea and Emys and as its sister either another clade (Clemmys guttata + Terrapene) or Terrapene alone. In contrast, nuclear genomic data yield conflicting results, depending on which genes are used. Either Clemmys guttata is revealed as sister to ((Emydoidea + Emys) + Actinemys) + Terrapene or Clemmys gut- tata is sister to Actinemys marmorata and these two species together are the sister group of (Emydoidea + Emys); Terra- pene appears then as sister to (Actinemys marmorata + Clemmys guttata) + (Emydoidea + Emys). The contradictory branching patterns depending from the selected loci are suggestive of lineage sorting problems. Ignoring the unclear phy- logenetic position of Actinemys marmorata, one recently proposed classification scheme placed Actinemys marmorata, Emydoidea blandingii, Emys orbicularis, and Emys trinacris in one genus (Emys), while another classification scheme treats Actinemys, Emydoidea, and Emys as distinct genera. -

(Testudines: Geoemydidae) from the Azov Sea Coast in the Crimea
Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 10(2) [General Section]: 27–29 (e129). Short Communication A record of the Balkan Stripe-necked Terrapin, Mauremys rivulata (Testudines: Geoemydidae) from the Azov Sea Coast in the Crimea 1Oleg V. Kukushkin and 2Daniel Jablonski 1Department of Herpetology, Zoological Institute of Russian Academy of Sciences, Universitetskaya Emb. 1, 199034 Saint Pe- tersburg, RUSSIA 2Department of Zoology, Comenius University in Bratislava, Mlynská dolina, Ilkovičova 6, 842 15 Bratislava, SLOVAKIA Keywords. Mauremys rivulata, first record, Crimea, Kerch peninsula, Azov Sea, overseas dispersal, occasional relocation Citation: Kukushkin O V, Jablonski D. 2016. A record of the Balkan Stripe-necked Terrapin, Mauremys rivulata (Testudines: Geomydidae) from the Azov Sea Coast in Crimea. Amphibian & Reptile Conservation 10(2) [General Section]: 27–29 (e129). Copyright: © 2016 Kukushkin and Jablonski. This is an open-access article distributed under the terms of the Creative Commons Attribution- NonCommercialNoDerivatives 4.0 International License, which permits unrestricted use for non-commercial and education purposes only, in any medium, provided the original author and the official and authorized publication sources are recognized and properly credited. The official and authorized publication credit sources, which will be duly enforced, are as follows: official journal titleAmphibian & Reptile Conservation; official journal website <amphibian-reptile-conservation.org>. Received: 03 September 2016; Accepted: 7 November 2016; Published: 30 November 2016 The Crimean herpetofauna comprises such true Eastern- limestone rocks on the abrasion-accumulative sea coast Mediterranean species as Mediodactylus kotschyi and below the lake (Fig. 1B). In general, the locality remains Zamenis situla (Sillero et al. 2014). The occurrence of typical of habitats of M. -

Introduction to Aquatic Turtle Care
Mississippi Map Turtle Introduction to Aquatic Turtle Care There are over 300 turtle species worldwide, including roughly 60 types of tortoise and 7 sea turtle species. Turtles are found on every Basking area: aquatic turtles need sufficient continent except Antarctica, living in a variety room to leave the water, dry their shells, of climates from the tropical regions of Cen- and regulate their temperature. tral and South America through the temper- Incandescent light fixture heats the ate parts of the U.S., with a few species in o- o) basking area (typically 85 95 to UVB light fixture for illumination; essential southern Canada. provide temperature gradient for vitamin synthesis in turtles held indoors The vast majority of turtles spend much of their lives in freshwater ponds, lakes and riv- ers. Although they are in the same family with North American pond and river turtles, box turtles of the U.S. and Mexico are primarily A filtration system terrestrial. to remove waste Tortoises are primarily terrestrial with differ- and prevent ill- ent habitat and diet requirements and are ness in your pet covered in a separate care sheet. turtle Underwater decorations: logs, driftwood, live or artificial plants, rock piles or other hiding places. Submersible thermometer to ensure water temperature is in the correct range, generally mid 70osF; varies with species, age and time of year A small to medium-sized aquarium (20-29 gallons) is ample for one adult of a smaller species Western painted turtle. Painted turtles (e.g., mud, musk). Larger species (sliders, cooters) may need tanks 100 gallons and larger. -

The Case of Deirocheline Turtles
bioRxiv preprint doi: https://doi.org/10.1101/556670; this version posted February 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Body coloration and mechanisms of colour production in Archelosauria: 2 The case of deirocheline turtles 3 Jindřich Brejcha1,2*†, José Vicente Bataller3, Zuzana Bosáková4, Jan Geryk5, 4 Martina Havlíková4, Karel Kleisner1, Petr Maršík6, Enrique Font7 5 1 Department of Philosophy and History of Science, Faculty of Science, Charles University, Viničná 7, Prague 6 2, 128 00, Czech Republic 7 2 Department of Zoology, Natural History Museum, National Museum, Václavské nám. 68, Prague 1, 110 00, 8 Czech Republic 9 3 Centro de Conservación de Especies Dulceacuícolas de la Comunidad Valenciana. VAERSA-Generalitat 10 Valenciana, El Palmar, València, 46012, Spain. 11 4 Department of Analytical Chemistry, Faculty of Science, Charles University, Hlavova 8, Prague 2, 128 43, 12 Czech Republic 13 5 Department of Biology and Medical Genetics, 2nd Faculty of Medicine, Charles University and University 14 Hospital Motol, V Úvalu 84, 150 06 Prague, Czech Republic 15 6 Department of Food Science, Faculty of Agrobiology, Food, and Natural Resources, Czech University of Life 16 Sciences, Kamýcká 129, Prague 6, 165 00, Czech Republic 17 7 Ethology Lab, Cavanilles Institute of Biodiversity and Evolutionary Biology, University of Valencia, C/ 18 Catedrátic José Beltrán Martinez 2, Paterna, València, 46980, Spain 19 Keywords: Chelonia, Trachemys scripta, Pseudemys concinna, nanostructure, pigments, chromatophores 20 21 Abstract 22 Animal body coloration is a complex trait resulting from the interplay of multiple colour-producing mechanisms. -

AN INTRODUCTION to Texas Turtles
TEXAS PARKS AND WILDLIFE AN INTRODUCTION TO Texas Turtles Mark Klym An Introduction to Texas Turtles Turtle, tortoise or terrapin? Many people get confused by these terms, often using them interchangeably. Texas has a single species of tortoise, the Texas tortoise (Gopherus berlanderi) and a single species of terrapin, the diamondback terrapin (Malaclemys terrapin). All of the remaining 28 species of the order Testudines found in Texas are called “turtles,” although some like the box turtles (Terrapene spp.) are highly terrestrial others are found only in marine (saltwater) settings. In some countries such as Great Britain or Australia, these terms are very specific and relate to the habit or habitat of the animal; in North America they are denoted using these definitions. Turtle: an aquatic or semi-aquatic animal with webbed feet. Tortoise: a terrestrial animal with clubbed feet, domed shell and generally inhabiting warmer regions. Whatever we call them, these animals are a unique tie to a period of earth’s history all but lost in the living world. Turtles are some of the oldest reptilian species on the earth, virtually unchanged in 200 million years or more! These slow-moving, tooth less, egg-laying creatures date back to the dinosaurs and still retain traits they used An Introduction to Texas Turtles | 1 to survive then. Although many turtles spend most of their lives in water, they are air-breathing animals and must come to the surface to breathe. If they spend all this time in water, why do we see them on logs, rocks and the shoreline so often? Unlike birds and mammals, turtles are ectothermic, or cold- blooded, meaning they rely on the temperature around them to regulate their body temperature. -
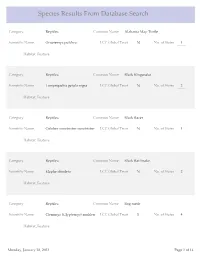
Species Results from Database Search
Species Results From Database Search Category Reptiles Common Name Alabama Map Turtle Scientific Name Graptemys pulchra LCC Global Trust N No. of States 1 Habitat_Feature Category Reptiles Common Name Black Kingsnake Scientific Name Lampropeltis getula nigra LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Black Racer Scientific Name Coluber constrictor constrictor LCC Global Trust N No. of States 1 Habitat_Feature Category Reptiles Common Name Black Rat Snake Scientific Name Elaphe obsoleta LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Bog turtle Scientific Name Clemmys (Glyptemys) muhlen LCC Global Trust Y No. of States 4 Habitat_Feature Monday, January 28, 2013 Page 1 of 14 Category Reptiles Common Name Broadhead Skink Scientific Name Eumeces laticeps LCC Global Trust N No. of States 5 Habitat_Feature Category Reptiles Common Name Coal Skink Scientific Name Eumeces anthracinus LCC Global Trust Y No. of States 8 Habitat_Feature Category Reptiles Common Name Common Five-lined Skink Scientific Name Eumeces fasciatus LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Common Map Turtle Scientific Name Graptemys geographica LCC Global Trust N No. of States 6 Habitat_Feature Category Reptiles Common Name Common Musk Turtle Scientific Name Sternotherus odoratus LCC Global Trust N No. of States 2 Habitat_Feature Monday, January 28, 2013 Page 2 of 14 Category Reptiles Common Name Common Ribbonsnake Scientific Name Thamnophis sauritus sauritus LCC Global Trust N No. of States 6 Habitat_Feature Category Reptiles Common Name Common Snapping Turtle Scientific Name Chelydra serpentina LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Corn snake Scientific Name Elaphe guttata guttata LCC Global Trust N No. -
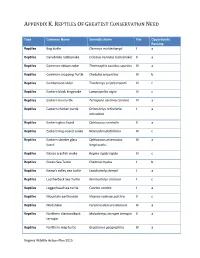
Reptiles of Greatest Conservation Need
APPENDIX K. REPTILES OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Reptiles Bog turtle Clemmys muhlenbergii I a Reptiles Canebrake rattlesnake Crotalus horridus (canebrake) II a Reptiles Common ribbonsnake Thamnophis sauritus sauritus IV a Reptiles Common snapping Turtle Chelydra serpentina IV b Reptiles Cumberland slider Trachemys scripta troostii III c Reptiles Eastern black kingsnake Lampropeltis nigra III c Reptiles Eastern box turtle Terrapene carolina carolina III a Reptiles Eastern chicken turtle Deirochelys reticularia I a reticularia Reptiles Eastern glass lizard Ophisaurus ventralis II a Reptiles Eastern hog-nosed snake Heterodon platirhinos IV c Reptiles Eastern slender glass Ophisaurus attenuatus IV a lizard longicaudus Reptiles Glossy crayfish snake Regina rigida rigida III c Reptiles Green Sea Turtle Chelonia mydas I b Reptiles Kemp's ridley sea turtle Lepidochelys kempii I a Reptiles Leatherback Sea Turtle Dermochelys coriacea I c Reptiles Loggerhead sea turtle Caretta caretta I a Reptiles Mountain earthsnake Virginia valeriae pulchra II c Reptiles Mudsnake Farancia abacura abacura IV a Reptiles Northern diamondback Malaclemys terrapin terrapin II a terrapin Reptiles Northern map turtle Graptemys geographica IV a Virginia Wildlife Action Plan 2015 APPENDIX K. REPTILES OF GREATEST CONSERVATION NEED Reptiles Northern pinesnake Pituophis melanoleucus I a melanoleucus Reptiles Queen snake Regina septemvittata IV a Reptiles Rainbow snake Farancia erytrogramma IV a erytrogramma Reptiles -

Membros Da Comissão Julgadora Da Dissertação
UNIVERSIDADE DE SÃO PAULO FACULDADE DE FILOSOFIA, CIÊNCIAS E LETRAS DE RIBEIRÃO PRETO PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA Evolution of the skull shape in extinct and extant turtles Evolução da forma do crânio em tartarugas extintas e viventes Guilherme Hermanson Souza Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo, como parte das exigências para obtenção do título de Mestre em Ciências, obtido no Programa de Pós- Graduação em Biologia Comparada Ribeirão Preto - SP 2021 UNIVERSIDADE DE SÃO PAULO FACULDADE DE FILOSOFIA, CIÊNCIAS E LETRAS DE RIBEIRÃO PRETO PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA Evolution of the skull shape in extinct and extant turtles Evolução da forma do crânio em tartarugas extintas e viventes Guilherme Hermanson Souza Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo, como parte das exigências para obtenção do título de Mestre em Ciências, obtido no Programa de Pós- Graduação em Biologia Comparada. Orientador: Prof. Dr. Max Cardoso Langer Ribeirão Preto - SP 2021 Autorizo a reprodução e divulgação total ou parcial deste trabalho, por qualquer meio convencional ou eletrônico, para fins de estudo e pesquisa, desde que citada a fonte. I authorise the reproduction and total or partial disclosure of this work, via any conventional or electronic medium, for aims of study and research, with the condition that the source is cited. FICHA CATALOGRÁFICA Hermanson, Guilherme Evolution of the skull shape in extinct and extant turtles, 2021. 132 páginas. Dissertação de Mestrado, apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto/USP – Área de concentração: Biologia Comparada. -

Ecology and Conservation Biology of the North American Wood Turtle (Glyptemys
Ecology and Conservation Biology of the North American Wood Turtle (Glyptemys insculpta) in the Central Appalachians A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Steven P. Krichbaum May 2018 © 2018 Steven P. Krichbaum. All Rights Reserved. 2 This dissertation titled Ecology and Conservation Biology of the North American Wood Turtle (Glyptemys insculpta) in the Central Appalachians by STEVEN P. KRICHBAUM has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Willem Roosenburg Professor of Biological Sciences Robert Frank Dean, College of Arts and Sciences 3 Abstract KRICHBAUM, STEVEN P., Ph.D., May 2018, Biological Sciences Ecology and Conservation Biology of the North American Wood Turtle (Glyptemys insculpta) in the Central Appalachians Director of Dissertation: Willem Roosenburg My study presents information on summer use of terrestrial habitat by IUCN “endangered” North American Wood Turtles (Glyptemys insculpta), sampled over four years at two forested montane sites on the southern periphery of the species’ range in the central Appalachians of Virginia (VA) and West Virginia (WV) USA. The two sites differ in topography, stream size, elevation, and forest composition and structure. I obtained location points for individual turtles during the summer, the period of their most extensive terrestrial roaming. Structural, compositional, and topographical habitat features were measured, counted, or characterized on the ground (e.g., number of canopy trees and identification of herbaceous taxa present) at Wood Turtle locations as well as at paired random points located 23-300m away from each particular turtle location. -

Conservation Biology of the European Pond Turtle Emys Orbicularis (L) in Italy 219-228 © Biologiezentrum Linz/Austria; Download Unter
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Stapfia Jahr/Year: 2000 Band/Volume: 0069 Autor(en)/Author(s): Zuffi Marco A. L. Artikel/Article: Conservation biology of the European pond turtle Emys orbicularis (L) in Italy 219-228 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Conservation biology of the European pond turtle Emys orbicularis (L) in Italy M.A.L. ZUFFI Abstract Key words The updated situation and knowledge Emys orbicularis, distribution, ecology, of the biology, ecology, behaviour and pro- conservation, Italy, tection of the European pond turtle, Emys orbicularis (L.) in Italy is presented and discussed in the light of conservation bio- logical issues. Stapfia 69, zugleich Kataloge des OÖ. Landesmuseums, Neue Folge Nr. 149 (2000), 219-228 219 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Introduction In this last decade, a "Big Bang" of interest in Italian populations of E. orbiculans enabled Populations of Emys orbicularis in Italy are to build up a consistent data set. Information distributed mainly in coastal areas and inter- on biometry (Zum & GARIBOLDI 1995a, b), nal plains. Most regions of Italy have been systematics (FRITZ & OBST 1995; FRITZ 1998), mapped, but in some cases the information is population structure (KELLER et al. 1998; KEL- incomplete (Fig. 1, Societas Herpetologica LER 1999), space usage (LEBBOROM & CHELA - Italica 1996). An uncomplete knowledge of ZZI 1991), reproductive biology (ZUFFI & habitat use leads to a biased view on the ODETTI 1998; ZUFFI et al. 1999; KELLER 1999), and thermal ecology (Dl TRAM & ZUFFI 1997), have become available. -

In AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): Species in Red = Depleted to the Point They May Warrant Federal Endangered Species Act Listing
Southern and Midwestern Turtle Species Affected by Commercial Harvest (in AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): species in red = depleted to the point they may warrant federal Endangered Species Act listing Common snapping turtle (Chelydra serpentina) – AR, GA, IA, KY, MO, OH, OK, SC, TX Florida common snapping turtle (Chelydra serpentina osceola) - FL Southern painted turtle (Chrysemys dorsalis) – AR Western painted turtle (Chrysemys picta) – IA, MO, OH, OK Spotted turtle (Clemmys gutatta) - FL, GA, OH Florida chicken turtle (Deirochelys reticularia chrysea) – FL Western chicken turtle (Deirochelys reticularia miaria) – AR, FL, GA, KY, MO, OK, TN, TX Barbour’s map turtle (Graptemys barbouri) - FL, GA Cagle’s map turtle (Graptemys caglei) - TX Escambia map turtle (Graptemys ernsti) – FL Common map turtle (Graptemys geographica) – AR, GA, OH, OK Ouachita map turtle (Graptemys ouachitensis) – AR, GA, OH, OK, TX Sabine map turtle (Graptemys ouachitensis sabinensis) – TX False map turtle (Graptemys pseudogeographica) – MO, OK, TX Mississippi map turtle (Graptemys pseuogeographica kohnii) – AR, TX Alabama map turtle (Graptemys pulchra) – GA Texas map turtle (Graptemys versa) - TX Striped mud turtle (Kinosternon baurii) – FL, GA, SC Yellow mud turtle (Kinosternon flavescens) – OK, TX Common mud turtle (Kinosternon subrubrum) – AR, FL, GA, OK, TX Alligator snapping turtle (Macrochelys temminckii) – AR, FL, GA, LA, MO, TX Diamond-back terrapin (Malaclemys terrapin) – FL, GA, LA, SC, TX River cooter (Pseudemys concinna) – AR, FL,