Specific Copb Transporter: Revising P1B-Type Atpase Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cellular Transport Notes About Cell Membranes
Cellular Transport Notes @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey About Cell Membranes • All cells have a cell membrane • Functions: – Controls what enters and exits the cell to maintain an internal balance called homeostasis TEM picture of a – Provides protection and real cell membrane. support for the cell @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey 1 About Cell Membranes (continued) 1.Structure of cell membrane Lipid Bilayer -2 layers of phospholipids • Phosphate head is polar (water loving) Phospholipid • Fatty acid tails non-polar (water fearing) • Proteins embedded in membrane Lipid Bilayer @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey Polar heads Fluid Mosaic love water Model of the & dissolve. cell membrane Non-polar tails hide from water. Carbohydrate cell markers Proteins @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey 2 About Cell Membranes (continued) • 4. Cell membranes have pores (holes) in it • Selectively permeable: Allows some molecules in and keeps other molecules out • The structure helps it be selective! Pores @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey Structure of the Cell Membrane Outside of cell Carbohydrate Proteins chains Lipid Bilayer Transport Protein Phospholipids Inside of cell (cytoplasm) @ 2011 Center for Pre-College Programs, New Jersey Institute of Technology, Newark, New Jersey 3 Types of Cellular Transport • Passive Transport celldoesn’tuseenergy 1. Diffusion 2. Facilitated Diffusion 3. Osmosis • Active Transport cell does use energy 1. -

Molecular Regulation and Physiology of the H ,K -Atpases in Kidney
Molecular Regulation and Physiology of the H؉,K؉-ATPases in Kidney Juan Codina and Thomas D. DuBose Jr Two H؉,K؉-adenosine triphosphatase (ATPase) proteins participate in K؉ absorption and ؉ ؉ ؉ H secretion in the renal medulla. Both the gastric (HK␣1) and colonic (HK␣2)H,K - ATPases have been localized and characterized by a number of techniques, and are known to be highly regulated in response to acid-base and electrolyte disturbances. Both ATPases are dimers of composition ␣/ that localize to the apical membrane and both interact with the tetraspanin protein CD63. Although CD63 interacts with the carboxy-terminus of the subunit of the colonic H؉,K؉-ATPase, it interacts with the -subunit of the gastric-␣ H؉,K؉-ATPase. Pharmacologically, both ATPases are distinct; for example, the gastric H؉,K؉-ATPase is inhibited by Sch-28080, but the colonic H؉,K؉-ATPase is inhibited by .ouabain (a classic inhibitor of the Na؉-pump) and is completely insensitive to Sch-28080 The ␣-subunit of the colonic H؉,K؉-ATPase is the only subunit of the X؉,K؉-ATPase superfamily that has 3 different splice variants that emerge by deletion or elongation of the amino-terminus. The messenger RNA and protein of one of these splice variants (HK␣2C)is specifically up-regulated in newborn rats and becomes undetectable in adult rats. There- fore, HK␣2, in addition to its role in potassium and acid-base homeostasis, appears to play a significant role in early growth and development. Finally, because chronic hypokalemia appears to be the most potent stimulus for upregulation of HK␣2, we propose that the HK␣2 participates importantly in the maintenance of chronic metabolic alkalosis. -

SERCA in Genesis of Arrhythmias: What We Already Know and What Is New?
Review 43 SERCA in genesis of arrhythmias: what we already know and what is new? Nilüfer Erkasap Department of Physiology, Medical Faculty, Eskiflehir Osmangazi University, Eskiflehir, Turkey ABSTRACT This review mainly focuses on the structure, function of the sarco(endo)plasmic reticulum calcium pump (SERCA) and its role in genesis of arrhythmias. SERCA is a membrane protein that belongs to the family of P-type ion translocating ATPases and pumps free cytosolic calcium into intracellular stores. Active transport of Ca2+ is achieved, according to the E1-E2 model, changing of SERCA structure by Ca2+. The affinity of Ca2+ -binding sites varies from high (E1) to low (E2). Three different SERCA genes were identified-SERCA1, SERCA2, and SERCA3. SERCA is mainly represented by the SERCA2a isoform in the heart. In heart muscle, during systole, depolarization triggers the release of Ca2+ from the sarcoplasmic reticulum (SR) and starts contraction. During diastole, muscle relaxation occurs as Ca2+ is again removed from cytosol, predominantly by accumulation into SR via the action of SERCA2a. The main regulator of SERCA2a is phospholamban and another regulator proteolipid of SERCA is sarcolipin. There are a lot of studies on the effect of decreased and/or increased SERCA activity in genesis of arrhythmia. Actually both decrease and increase of SERCA activity in the heart result in some pathological mechanisms such as heart failure and arrhythmia. (Anadolu Kardiyol Derg 2007: 7 Suppl 1; 43-6) Key words: sarco(endo)plasmic reticulum, SERCA, arrhythmia, calcium channels Introduction from cytosol, predominantly by accumulation into sarcoplasmic reticulum via the action of sarco(endo)plasmic reticulum Cardiac physiology is a major area of research in basic and Ca ATPase (SERCA). -

Passive and Active Transport
Passive and Active Transport 1. Thermodynamics of transport 2. Passive-mediated transport 3. Active transport neuron, membrane potential, ion transport Membranes • Provide barrier function – Extracellular – Organelles • Barrier can be overcome by „transport proteins“ – To mediate transmembrane movements of ions, Na+, K+ – Nutrients, glucose, amino acids etc. – Water (aquaporins) 1) Thermodynamics of Transport • Aout <-> Ain (ressembles a chemical equilibration) o‘ • GA - G A = RT ln [A] • ∆GA = GA(in) - GA(out) = RT ln ([A]in/[A]out) • GA: chemical potential of A o‘ • G A: chemical potential of standard state of A • If membrane has a potential, i.e., plasma membrane: -100mV (inside negative) then GA is termed the electrochemical potential of A Two types of transport across a membrane: o Nonmediated transport occurs by passive diffusion, i.e., O2, CO2 driven by chemical potential gradient, i.e. cannot occur against a concentration gradient o Mediated transport occurs by dedicated transport proteins 1. Passive-mediated transport/facilitated diffusion: [high] -> [low] 2. Active transport: [low] -> [high] May require energy in form of ATP or in form of a membrane potential 2) Passive-mediated transport Substances that are too large or too polar to diffuse across the bilayer must be transported by proteins: carriers, permeases, channels and transporters A) Ionophores B) Porins C) Ion Channels D) Aquaporins E) Transport Proteins A) Ionophores Organic molecules of divers types, often of bacterial origin => Increase the permeability of a target membrane for ions, frequently antibiotic, result in collapse of target membrane potential by ion equilibration 1. Carrier Ionophore, make ion soluble in membrane, i.e. valinomycin, 104 K+/sec 2. -

The Electrochemical Gradient of Protons and Its Relationship to Active Transport in Escherichia Coli Membrane Vesicles
Proc. Natl. Acad. Sci. USA Vol. 73, No. 6, pp. 1892-1896, June 1976 Biochemistry The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles (flow dialysis/membrane potential/energy transduction/lipophilic cations/weak acids) SOFIA RAMOS, SHIMON SCHULDINER*, AND H. RONALD KABACK The Roche Institute of Molecular Biology, Nutley, New Jersey 07110 Communicated by B. L. Horecker, March 17, 1976 ABSTRACT Membrane vesicles isolated from E. coli gen- presence of valinomycin), a respiration-dependent membrane erate a trans-membrane proton gradient of 2 pH units under potential (AI, interior negative) of approximately -75 mV in appropriate conditions when assayed by flow dialysis. Using E. coli membrane vesicles has been documented (6, 13, 14). the distribution of weak acids to measure the proton gradient (ApH) and the distribution of the lipophilic cation triphenyl- Moreover it has been shown that the potential causes the ap- methylphosphonium to measure the electrical potential across pearance of high affinity binding sites for dansyl- and azido- the membrane (AI), the vesicles are shown to generate an phenylgalactosides on the outer surface of the membrane (4, electrochemical proton gradient (AiH+) of approximately -180 15) and that the potential is partially dissipated as a result of mV at pH 5.5 in the presence of ascorbate and phenazine lactose accumulation (6). Although these findings provide ev- methosulfate, the major component of which is a ApH of about idence for the chemiosmotic hypothesis, it has also been dem- -110 mV. As external pH is increased, ApH decreases, reaching o at pH 7.5 and above, while AI remains at about -75 mV and onstrated (6, 16) that vesicles are able to accumulate lactose and internal pH remains at pH 7.5. -

Biology Passive & Active Transport April 30, 2020
High School Science Virtual Learning Biology Passive & Active Transport April 30, 2020 High School General Biology Lesson: Passive & Active Transport Objective/Learning Target: Students will understand how passive and active transports work. Bell Ringer Activity 1. If someone is being active what does that mean? 2. If someone is being passive what does that mean? Bell Ringer Answers 1. If someone is being active that means they are marked by energetic activity. 2. If someone is being passive they are accepting what happens to others without an active response. Keep these definitions in mind as we discuss the differences between what active and passive transport are in biology. Let’s Get Started! Lesson Activity: Directions: 1. Watch this video. 2. Create a Venn Diagram like the one you see here ---> 3. Compare and contrast Active and Passive Transport by the information you learn from the video. Lesson Questions Answers Venn Diagram Examples: Practice Questions 1. What is passive transport? 2. What is active transport? 3. What is the difference between diffusion and osmosis? 4. What is the difference between endocytosis and exocytosis? 5. What is the differences between facilitated diffusion and active transport by a protein pump? Answers to Practice Questions 1. Passive transport is the movement of materials across the cell membrane without using cellular energy. 2. Active transport is the movement of materials against a concentration difference; it requires energy. 3. In diffusion, both solvent and solute particles are free to move; however, in osmosis only water molecules cross the semipermeable membrane. Answers to Practice Questions Continued 4. -
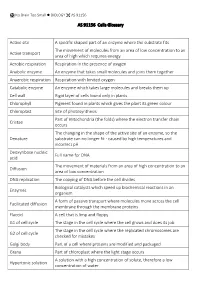
AS 91156 Cells Glossary Active Site a Specific Shaped Part of an Enzyme Where the Substrate Fits Active Transport the Movement
No Brain Too Small BIOLOGY AS 91156 AS 91156 Cells Glossary Active site A specific shaped part of an enzyme where the substrate fits The movement of molecules from an area of low concentration to an Active transport area of high which requires energy Aerobic respiration Respiration in the presence of oxygen Anabolic enzyme An enzyme that takes small molecules and joins them together Anaerobic respiration Respiration with limited oxygen Catabolic enzyme An enzyme which takes large molecules and breaks them up Cell wall Rigid layer of cells found only in plants Chlorophyll Pigment found in plants which gives the plant its green colour Chloroplast Site of photosynthesis Part of mitochondria (the folds) where the electron transfer chain Cristae occurs The changing in the shape of the active site of an enzyme, so the Denature substrate can no longer fit - caused by high temperatures and incorrect pH Deoxyribose nucleic Full name for DNA acid The movement of materials from an area of high concentration to an Diffusion area of low concentration DNA replication The copying of DNA before the cell divides Biological catalysts which speed up biochemical reactions in an Enzymes organism A form of passive transport where molecules move across the cell Facilitated diffusion membrane through the membrane proteins Flaccid A cell that is limp and floppy G1 of cell cycle The stage in the cell cycle where the cell grows and does its job The stage in the cell cycle where the replicated chromosomes are G2 of cell cycle checked for mistakes Golgi body Part -

Ca2+-Atpase Molecules As a Calcium-Sensitive Membrane-Endoskeleton of Sarcoplasmic Reticulum
International Journal of Molecular Sciences Article Ca2+-ATPase Molecules as a Calcium-Sensitive Membrane-Endoskeleton of Sarcoplasmic Reticulum Jun Nakamura 1,*, Yuusuke Maruyama 1, Genichi Tajima 2, Yuto Komeiji 1, Makiko Suwa 3 and Chikara Sato 1,* 1 Health and Medical Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Central 6, 1-1-1 Higashi, Tsukuba, Ibaraki 305-8566, Japan; [email protected] (Y.M.); [email protected] (Y.K.) 2 Institute for Excellence in Higher Education, Tohoku University, 41 Kawauchi, Aoba-ku, Sendai, Miyagi 980-8576, Japan; [email protected] 3 Biological Science Course, Graduate School of Science and Engineering, Aoyama Gakuin University, 5-10-1 Fuchinobe, Chuou-ku, Sagamihara, Kanagawa 252-5258, Japan; [email protected] * Correspondence: [email protected] (J.N.); [email protected] (C.S.) Abstract: The Ca2+-transport ATPase of sarcoplasmic reticulum (SR) is an integral, transmembrane protein. It sequesters cytoplasmic calcium ions released from SR during muscle contraction, and causes muscle relaxation. Based on negative staining and transmission electron microscopy of SR vesicles isolated from rabbit skeletal muscle, we propose that the ATPase molecules might also be a calcium-sensitive membrane-endoskeleton. Under conditions when the ATPase molecules scarcely transport Ca2+, i.e., in the presence of ATP and ≤ 0.9 nM Ca2+, some of the ATPase particles on the SR vesicle surface gathered to form tetramers. The tetramers crystallized into a cylindrical helical array in some vesicles and probably resulted in the elongated protrusion that extended from some round SRs. -

ATP Synthase: Structure,Abstract: Functionlet F Denote a Eld and and Let Inhibitionv Denote a Vector Space Over F with Nite Positive Dimension
Spec. Matrices 2019; 7:1–19 Research Article Open Access Kazumasa Nomura* and Paul Terwilliger BioMol Concepts 2019; 10: 1–10 Self-dual Leonard pairs Research Article Open Access https://doi.org/10.1515/spma-2019-0001 Prashant Neupane*, Sudina Bhuju, Nita Thapa,Received Hitesh May 8, 2018; Kumar accepted Bhattarai September 22, 2018 ATP Synthase: Structure,Abstract: FunctionLet F denote a eld and and let InhibitionV denote a vector space over F with nite positive dimension. Consider a pair A, A∗ of diagonalizable F-linear maps on V, each of which acts on an eigenbasis for the other one in an irreducible tridiagonal fashion. Such a pair is called a Leonard pair. We consider the self-dual case in which https://doi.org/10.1515/bmc-2019-0001 there exists an automorphism of the endomorphism algebra of V that swaps A and A∗. Such an automorphism phosphate (Pi), along with considerable release of energy. received September 18, 2018; accepted December 21, 2018. is unique, and called the duality A A∗. In the present paper we give a comprehensive description of this ADP can absorb energy and regain↔ the group to regenerate duality. In particular, we display an invertible F-linear map T on V such that the map X TXT− is the duality Abstract: Oxidative phosphorylation is carried out by an ATP molecule to maintain constant ATP concentration. → A A∗. We express T as a polynomial in A and A∗. We describe how T acts on ags, decompositions, five complexes, which are the sites for electron transport↔ Other than supporting almost all the cellular and ATP synthesis. -

Living Environment Vocabulary by Prentice Hall 2001 Review Book Unit
Living Environment Vocabulary By Prentice Hall 2001 Review Book Unit Similarities and Topic 1 Differences Among Living Organisms cell the basic unit of structure and function that makes up all organisms metabolism all the chemical reactions that occur within the cells of an organism homeostasis the ability of an organism to maintain a stable internal environment even when the external environment changes reproduction the process by which organisms produce new organisms of the same type cell respiration the process in which nutrients are broken apart, releasing the chemical energy stored in them synthesis a life process that involves combining simple substances into more complex substances organic term used to describe molecules that contain both hydrogen and carbon inorganic a type of molecule that does not contain both carbon and hydrogen but can contain any other combination of elements organelle a structure within the cell that carries out a specific function tissues a group of specialized cells that perform a specific function organ a body structure made of different kinds of tissues combined to perform a specific function organ system several organs that work together to perform a major function in the body cytoplasm the jellylike substance that is between the cell membrane and the nucleus and that contains specialized structures nucleus a large structure within a cell that controls the cell’s metabolism and stores genetic information, including chromosomes and DNA vacuoles storage sacs within the cytoplasm of a cell that may contain -

Gastric H,K-Atpase As a Drug Target
UCLA UCLA Previously Published Works Title Gastric H,K-ATPase as a drug target Permalink https://escholarship.org/uc/item/1g5606x9 Journal Digestive Diseases and Sciences, 51(5) ISSN 0163-2116 Authors Shin, Jai M Sachs, G Publication Date 2006-05-01 Peer reviewed eScholarship.org Powered by the California Digital Library University of California The Gastric H,K-ATPase as a Drug Target Jai Moo Shin and George Sachs* Department of Physiology and Medicine, University of California at Los Angeles, and VA Greater Los Angeles Healthcare System, Los Angeles, California, CA90073, USA * To whom correspondence should be addressed: at Membrane Biology Laboratory, VA Greater Los Angeles Healthcare System, 11301 Wilshire Blvd., Bldg. 113, Rm. 324, Los Angeles, CA 90073 Tel: (310) 268-4672 Fax: (310) 312-9478 e-mail: [email protected] 1 Introduction Gastric acid is secreted by parietal cells in the stomach. These have two known acid stimulatory receptors the H2-receptor and the muscarinic M3 receptor. Gastrin, the major endocrine activator of acid secretion, exerts its action via release of histamine from the ECL cell as does pituitary adenylate cyclase activating peptide (PACAP), a neural mediator of activation of acid secretion. Antagonists of the former two stimulants inhibit gastric acid secretion. Cholinergic receptor antagonists have many side effects and are relatively weak inhibitors at therapeutic doses as compared toH2-receptor antagonists. These drugs were widely developed in the 1970’s and 1980’s and became the first really useful medications for healing of peptic ulcers. However, although good for healing peptic ulcers, they were less effective in treatment of erosive esophagitis. -

Conformational Model of Active Transport John H
Proceedings of the National Academy of Sciences Vol. 67, No. 2, pp. 550-559, October 1970 Conformational Model of Active Transport John H. Young*, George A. Blondin, G. Vanderkooi, and D. E. Green INSTITUTE FOR ENZYME RESEARCH AND THEORETICAL CHEMISTRY INSTITUTE UNIVERSITY OF WISCONSIN, MADISON 53706 Read as an Invited Paper on the Conformational Basis of an Energy Transduction, April 29, 1970 Abstract. A model of active transport of monovalent cations in mitochondria is developed. The model is based on the coupling of electron transfer to the generation of a metastable protein conformation which in turn leads to the generation of an asymmetric surface charge, a membrane potential, and a redis- tribution of diffusible ions across the inner mitochondrial membrane. The ions at all times move spontaneously down an electrochemical potential gradient in this model so that there is no need to invoke the concept of an ion pump. It is shown that a wide variety of experimental facts can be rationalized in terms of the present model. In seeking an explanation for active transport, two mutually exclusive alterna- tives present themselves-either the ions move spontaneously down an electro- chemical potential gradient or they do not. Whereas many investigators in the field of active transport believe that ions are pumped against an electro- chemical potential gradient, the model we will present involves no ion pumps but only the spontaneous movement of ions down an electrochemical potential gradient. We will consider only one active transport system, the mitochondrion, and we will consider only monovalent cation translocation in the mitochondrion. Even for this special case we cannot yet claim to have a complete explanation of active transport.