Genetics of Hypertension Paul N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CADASIL Testing
Lab Management Guidelines V1.0.2020 CADASIL Testing MOL.TS.144.A v1.0.2020 Introduction CADASIL testing is addressed by this guideline. Procedures addressed The inclusion of any procedure code in this table does not imply that the code is under management or requires prior authorization. Refer to the specific Health Plan's procedure code list for management requirements. Procedures addressed by this Procedure codes guideline NOTCH3 Known Familial Mutation 81403 Analysis NOTCH3 Targeted Sequencing 81406 NOTCH3 Deletion/Duplication Analysis 81479 What is CADASIL Definition CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy) is an adult-onset form of cerebrovascular disease. There are no generally accepted clinical diagnostic criteria for CADASIL and symptoms vary among affected individuals. Signs and symptoms Typical signs and symptoms include1,2,3 Transient ischemic attacks and ischemic stroke, occurs at a mean age of 47 years (age range 20-70 years), in most cases without conventional vascular risk factors cognitive disturbance, primarily affecting executive function, may start as early as age 35 years psychiatric or behavioral abnormalities migraine with aura, occurs with a mean age of onset of 30 years (age range 6-48 years), and Less common symptoms include: © 2020 eviCore healthcare. All Rights Reserved. 1 of 7 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Lab Management Guidelines V1.0.2020 recurrent seizures with onset in middle age, usually secondary to stroke acute encephalopathy, with a mean age of onset of 42 years Life expectancy for men with CADASIL is reduced by approximately five years and for women by 1 to 2 years.4 Diagnosis Brain Magnetic Resonance Imaging (MRI) findings include T2-signal-abnormalities in the white matter of the temporal pole and T2-signal-abnormalities in the external capsule and corpus callosum.1,2 CADASIL is suspected in an individual with the clinical signs and MRI findings. -

The National Economic Burden of Rare Disease Study February 2021
Acknowledgements This study was sponsored by the EveryLife Foundation for Rare Diseases and made possible through the collaborative efforts of the national rare disease community and key stakeholders. The EveryLife Foundation thanks all those who shared their expertise and insights to provide invaluable input to the study including: the Lewin Group, the EveryLife Community Congress membership, the Technical Advisory Group for this study, leadership from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH), the Undiagnosed Diseases Network (UDN), the Little Hercules Foundation, the Rare Disease Legislative Advocates (RDLA) Advisory Committee, SmithSolve, and our study funders. Most especially, we thank the members of our rare disease patient and caregiver community who participated in this effort and have helped to transform their lived experience into quantifiable data. LEWIN GROUP PROJECT STAFF Grace Yang, MPA, MA, Vice President Inna Cintina, PhD, Senior Consultant Matt Zhou, BS, Research Consultant Daniel Emont, MPH, Research Consultant Janice Lin, BS, Consultant Samuel Kallman, BA, BS, Research Consultant EVERYLIFE FOUNDATION PROJECT STAFF Annie Kennedy, BS, Chief of Policy and Advocacy Julia Jenkins, BA, Executive Director Jamie Sullivan, MPH, Director of Policy TECHNICAL ADVISORY GROUP Annie Kennedy, BS, Chief of Policy & Advocacy, EveryLife Foundation for Rare Diseases Anne Pariser, MD, Director, Office of Rare Diseases Research, National Center for Advancing Translational Sciences (NCATS), National Institutes of Health Elisabeth M. Oehrlein, PhD, MS, Senior Director, Research and Programs, National Health Council Christina Hartman, Senior Director of Advocacy, The Assistance Fund Kathleen Stratton, National Academies of Science, Engineering and Medicine (NASEM) Steve Silvestri, Director, Government Affairs, Neurocrine Biosciences Inc. -

Cadasil Pathogenesis, Clinical and Radiological Findings and Treatment
View and review Arq Neuropsiquiatr 2010;68(2):287-299 Cadasil Pathogenesis, clinical and radiological findings and treatment Charles André ABSTRACT Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common genetic cause of ischemic strokes and a most important model for the study of subcortical vascular dementia. This unrelentlessly progressive disease affects many hundreds of families all over the world but is not well studied in Brazil. This manuscript reviews pathogenetic, clinical, radiological and therapeutic features of CADASIL. The causal mutations are now very well known, but the same can not be said about its intimate pathogenetic mechanisms. The variable clinical presentation should lead physicians to actively pursue the diagnosis in many settings and to more thouroughly investigate family history in first degree relatives. A rational approach to genetic testing is however needed. Treatment of CADASIL is still largely empiric. High- quality therapeutic studies involving medications and cognitive interventions are strongly needed in CADASIL. Key words: CADASIL, etiology, genetics, diagnosis, therapeutics. CADASIL: patogênese, achados clínicos e radiológicos e tratamento RESUMO CADASIL é a causa genética mais freqüente de infartos cerebrais e constitui modelo importante de estudo de demências vasculares subcorticais. De natureza inexoravelmente progressiva, afeta milhares de pessoas em todo o mundo. Sua importância é pouco reconhecida entre nós, o que nos levou à presente revisão dos principais aspectos patogenéticos, clínicos, neuroradiológicos e terapêuticos da doença. As mutações causais são hoje bem conhecidas, mas os mecanismos patogenéticos íntimos ainda permanecem misteriosos. A apresentação clínica variável deve fazer com que os médicos considerem o diagnóstico em vários contextos clínicos e investiguem de forma mais extensa que o usual a história familial deparentes de primeiro grau. -

Prevalence and Incidence of Rare Diseases: Bibliographic Data
Number 1 | January 2019 Prevalence and incidence of rare diseases: Bibliographic data Prevalence, incidence or number of published cases listed by diseases (in alphabetical order) www.orpha.net www.orphadata.org If a range of national data is available, the average is Methodology calculated to estimate the worldwide or European prevalence or incidence. When a range of data sources is available, the most Orphanet carries out a systematic survey of literature in recent data source that meets a certain number of quality order to estimate the prevalence and incidence of rare criteria is favoured (registries, meta-analyses, diseases. This study aims to collect new data regarding population-based studies, large cohorts studies). point prevalence, birth prevalence and incidence, and to update already published data according to new For congenital diseases, the prevalence is estimated, so scientific studies or other available data. that: Prevalence = birth prevalence x (patient life This data is presented in the following reports published expectancy/general population life expectancy). biannually: When only incidence data is documented, the prevalence is estimated when possible, so that : • Prevalence, incidence or number of published cases listed by diseases (in alphabetical order); Prevalence = incidence x disease mean duration. • Diseases listed by decreasing prevalence, incidence When neither prevalence nor incidence data is available, or number of published cases; which is the case for very rare diseases, the number of cases or families documented in the medical literature is Data collection provided. A number of different sources are used : Limitations of the study • Registries (RARECARE, EUROCAT, etc) ; The prevalence and incidence data presented in this report are only estimations and cannot be considered to • National/international health institutes and agencies be absolutely correct. -

Insurance and Advance Pay Test Requisition
Insurance and Advance Pay Test Requisition (2021) For Specimen Collection Service, Please Fax this Test Requisition to 1.610.271.6085 Client Services is available Monday through Friday from 8:30 AM to 9:00 PM EST at 1.800.394.4493, option 2 Patient Information Patient Name Patient ID# (if available) Date of Birth Sex designated at birth: 9 Male 9 Female Street address City, State, Zip Mobile phone #1 Other Phone #2 Patient email Language spoken if other than English Test and Specimen Information Consult test list for test code and name Test Code: Test Name: Test Code: Test Name: 9 Check if more than 2 tests are ordered. Additional tests should be checked off within the test list ICD-10 Codes (required for billing insurance): Clinical diagnosis: Age at Initial Presentation: Ancestral Background (check all that apply): 9 African 9 Asian: East 9 Asian: Southeast 9 Central/South American 9 Hispanic 9 Native American 9 Ashkenazi Jewish 9 Asian: Indian 9 Caribbean 9 European 9 Middle Eastern 9 Pacific Islander Other: Indications for genetic testing (please check one): 9 Diagnostic (symptomatic) 9 Predictive (asymptomatic) 9 Prenatal* 9 Carrier 9 Family testing/single site Relationship to Proband: If performed at Athena, provide relative’s accession # . If performed at another lab, a copy of the relative’s report is required. Please attach detailed medical records and family history information Specimen Type: Date sample obtained: __________ /__________ /__________ 9 Whole Blood 9 Serum 9 CSF 9 Muscle 9 CVS: Cultured 9 Amniotic Fluid: Cultured 9 Saliva (Not available for all tests) 9 DNA** - tissue source: Concentration ug/ml Was DNA extracted at a CLIA-certified laboratory or a laboratory meeting equivalent requirements (as determined by CAP and/or CMS)? 9 Yes 9 No 9 Other*: If not collected same day as shipped, how was sample stored? 9 Room temp 9 Refrigerated 9 Frozen (-20) 9 Frozen (-80) History of blood transfusion? 9 Yes 9 No Most recent transfusion: __________ /__________ /__________ *Please contact us at 1.800.394.4493, option 2 prior to sending specimens. -

New Insights Into the Diagnosis and Treatment of Single-Gene Disorders Associated with Cryptogenic Ischemic Stroke
New Insights Into the Diagnosis and Treatment of Single-Gene Disorders Associated With Cryptogenic Ischemic Stroke A Continuing Medical Education Monograph Release date: September 30, 2010 Expiration date: September 30, 2013 Editor Contributors Katherine B. Sims, MD Mark J. Alberts, MD, FAHA Louis R. Caplan, MD Massachusetts General Hospital Northwestern University Beth Israel Deaconess Medical Center Harvard Medical School Feinberg School of Medicine Harvard Medical School Boston, Massachusetts Northwestern Memorial Hospital Boston, Massachusetts Chicago, Illinois This activity is jointly sponsored by the University of Kentucky College of Medicine and CE Health Sciences Inc. New Insights Into the Diagnosis and Treatment of Single-Gene Disorders Associated With Cryptogenic Ischemic Stroke A Continuing Medical Education Monograph Editor Katherine B. Sims, MD Director, Developmental Neurogenetics Clinic Director, Neurogenetics DNA Diagnostic Laboratory Massachusetts General Hospital Associate Professor of Neurology Harvard Medical School Boston, Massachusetts Contributors Mark J. Alberts, MD, FAHA Professor of Neurology Section Chief, Stroke and Cerebrovascular Disease Northwestern University, Feinberg School of Medicine Director, Stroke Program Northwestern Memorial Hospital Chicago, Illinois Louis R. Caplan, MD Beth Israel Deaconess Medical Center Professor of Neurology Harvard Medical School Boston, Massachusetts To receive CME credit for this activity, please review the material in full and complete the online posttest and evaluation form at www.CECentral.com/getcredit (activity code MEN09182). A printable statement of credit will be issued upon successful completion of the required forms. 3 Accreditation This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the University of Kentucky College of Medicine and CE Health Sciences Inc. -

A Pure Model for Studying Cerebral Small Vessel Disease
From Department of Neurobiology, Care Sciences and Society (NVS) Karolinska Institutet, Stockholm, Sweden CADASIL: A PURE MODEL FOR STUDYING CEREBRAL SMALL VESSEL DISEASE Mahmod Panahi Stockholm 2019 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by Printed by E-Print AB 2018 © Mahmod Panahi, 2019 ISBN 978-91-7831-485-0 CADASIL: A pure model for studying cerebral small vessel disease THESIS FOR DOCTORAL DEGREE (Ph.D.) By Mahmod Panahi Principal Supervisor: Opponent: Homira Behbahani Christof Haffner Karolinska Institutet Ludwig-Maximilians-University Department of Neurobiology, Care Sciences and Department of Biochemistry Society (NVS) Division of Stroke and Dementia Research Division of Neurogeriatrics Examination Board: Co-supervisor(s): Ewa Ehrenborg Matti Viitanen Karolinska Institutet Karolinska Institutet Department of Medicine Department of Neurobiology, Care Sciences and Society (NVS) Johan Lökk Division of Clinical geriatrics Karolinska Institutet Department of Neurobiology, Care Sciences and Taher Darreh-Shori Society (NVS) Karolinska Institutet Division of Clinical geriatrics Department of Neurobiology, Care Sciences and Society (NVS) Katarina Nägga Division of Clinical geriatrics Linköping University Department of Clinical and Experimental Medicine Division of Neuro and Inflammation Sciences ABSTRACT Cerebral autosomal dominant arteriopathy with subcortical infarct and leukoencephalopathy (CADASIL) is caused by a mutation on the NOTCH3 gene. The pathological driver behind this disease is the loss of vascular smooth muscle cells (VSMCs) in small blood vessels and subsequent fibrotic thickening of the vessel, causing stenosis. Although a great deal of knowledge has been accumulated through CADASIL research, more information is needed to fully grasp the pathological mechanisms as well as understand disease progression. -

Genetic Testing: a Primer for Non-Geneticists
Genetic testing A primer for non-geneticists John Pappas, MD Gilad Evrony, MD PhD Heather Lau, MD Ellen Moran, MS Center for Human DC T 07/29/2020 Genetics & Genomics Department of Neurology Center for Human Genetics & Genomics Director: Aravinda Chakravarti Advancing human genetics programs in research, education, and patient care. E-mail us to sign up for our mailing list: [email protected] https://med.nyu.edu/centers-programs/human-genetics-genomics/ Educational programs, seminars, Genetic Medicine Colloquium. DC T 07/29/2020 A mini-seminar in 3 parts Part 1: The Basics Part 2: Understanding genetic test reports and basic counseling Part 3: Genomics and exome sequencing Center for Human Genetics & Genomics DC T 07/29/2020 GE A mini-seminar in 3 parts Part 1: The Basics Part 2: Understanding genetic test reports and basic counseling Part 3: Genomics and exome sequencing Center for Human Genetics & Genomics DC T 07/29/2020 GE Learning objectives Part 1: The Basics 1. Recognize general principles of when to consider a genetic disease. 2. Understand the available genetic testing methods, and the limitations of each. 3. Learn when and how to order genetic testing in your clinic, versus when to refer to clinical genetics/neurogenetics. Center for Human Genetics & Genomics DC T 07/29/2020 GE Disclosures John Pappas: None Gilad Evrony: None Heather Lau Consulting: Amicus, ASPA Therapeutics, Genzyme/Sanofi, Prevail, Takeda/Shire, Ultragenyx Ellen Moran: None Center for Human Genetics & Genomics DC T 07/29/2020 GE When to consider a genetic syndrome or disease? General for all age groups 1. -

Cerebral Autosomal Dominant Arteriopathy With
Di Donato et al. BMC Medicine (2017) 15:41 DOI 10.1186/s12916-017-0778-8 Vascular Dementia REVIEW Open Access Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects Ilaria Di Donato1, Silvia Bianchi1, Nicola De Stefano1, Martin Dichgans2,3, Maria Teresa Dotti1, Marco Duering2, Eric Jouvent4,5,6, Amos D. Korczyn7, Saskia A. J. Lesnik-Oberstein8, Alessandro Malandrini1, Hugh S. Markus9, Leonardo Pantoni10, Silvana Penco11, Alessandra Rufa1, Osman Sinanović12, Dragan Stojanov13 and Antonio Federico1* Abstract Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common and best known monogenic small vessel disease. Here, we review the clinical, neuroimaging, neuropathological, genetic, and therapeutic aspects based on the most relevant articles published between 1994 and 2016 and on the personal experience of the authors, all directly involved in CADASIL research and care. We conclude with some suggestions that may help in the clinical practice and management of these patients. Keywords: CADASIL, Small vessel disease, NOTCH 3, Vascular dementia, Genetics Background muscle cells and pericytes [1]. Thousands of families Cerebral small vessel disease (SVD) is an important with CADASIL have now been diagnosed worldwide in cause of stroke, cognitive impairment, and mood disor- many different ethnic groups. The disorder is often over- ders in the elderly. Besides the common sporadic forms, looked and misdiagnosed. Its minimum prevalence has mostly related to age and hypertension, a minority of been estimated between 2 and 5 in 100,000 but may vary SVD has a monogenic cause, among which the most between populations [2–5]. -

Genetic Testing Medical Policy – Genetics
Genetic Testing Medical Policy – Genetics Please complete all appropriate questions fully. Suggested medical record documentation: • Current History & Physical • Progress Notes • Family Genetic History • Genetic Counseling Evaluation *Failure to include suggested medical record documentation may result in delay or possible denial of request. PATIENT INFORMATION Name: Member ID: Group ID: PROCEDURE INFORMATION Genetic Counseling performed: c Yes c No **Please check the requested analyte(s), identify number of units requested, and provide indication/rationale for testing. 81400 Molecular Pathology Level 1 Units _____ c ACADM (acyl-CoA dehydrogenase, C-4 to C-12 straight chain, MCAD) (e.g., medium chain acyl dehydrogenase deficiency), K304E variant _____ c ACE (angiotensin converting enzyme) (e.g., hereditary blood pressure regulation), insertion/deletion variant _____ c AGTR1 (angiotensin II receptor, type 1) (e.g., essential hypertension), 1166A>C variant _____ c BCKDHA (branched chain keto acid dehydrogenase E1, alpha polypeptide) (e.g., maple syrup urine disease, type 1A), Y438N variant _____ c CCR5 (chemokine C-C motif receptor 5) (e.g., HIV resistance), 32-bp deletion mutation/794 825del32 deletion _____ c CLRN1 (clarin 1) (e.g., Usher syndrome, type 3), N48K variant _____ c DPYD (dihydropyrimidine dehydrogenase) (e.g., 5-fluorouracil/5-FU and capecitabine drug metabolism), IVS14+1G>A variant _____ c F13B (coagulation factor XIII, B polypeptide) (e.g., hereditary hypercoagulability), V34L variant _____ c F2 (coagulation factor 2) (e.g., -

Genes for Stroke
EDITORIAL 1229 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2004.036202 on 16 August 2004. Downloaded from Stroke genes its sensitivity vary from 50% to over ....................................................................................... 90%.17 The identification of magnetic reso- nance imaging (MRI) features, highly Genes for stroke suggestive of CADASIL, has greatly increased recognition of the disease.19 20 H Markus Confluent involvement of the anterior temporal pole (fig 1) is rare in sporadic ................................................................................... cerebral small vessel disease, but is Identifying the genes involved in multifactorial stroke present in over 90% of patients with CADASIL.17 19 20 Involvement of the external capsule is also common but alf the risk of ischaemic stroke of prevalence are difficult because of less specific. In contrast to sporadic remains unexplained by conven- significant under reporting, but a mini- small vessel disease,17 corpus callosum Htional risk factors1 and genetic mum prevalence of 1 in 100 000 has involvement may occur.21 This can lead predisposition has been widely specu- been estimated in south east England to misdiagnosis as multiple sclerosis. lated to account for some of this (unpublished data). However, despite The combination of improved MRI unexplained risk.2 Although significant its relative frequency, recent studies diagnosis and wider availability of progress has been made unravelling the have shown that CADASIL accounts genetic testing has led to both increased basis of single gene stroke disorders, for only a minority of patients with diagnosis of CADASIL and the apprecia- identifying the underlying genes for small vessel disease stroke on a popula- tion that the phenotype is much more common or multifactorial stroke, for tion basis.11 diverse than originally described. -
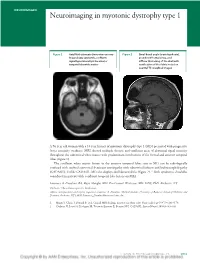
Neuroimaging in Myotonic Dystrophy Type 1
NEUROIMAGES Neuroimaging in myotonic dystrophy type 1 Figure 1 Axial fluid-attenuated inversion recovery Figure 2 Small basal angle (craniokyphosis), image shows symmetric, confluent prominent frontal sinus, and signal hyperintensity in the anterior diffuse thickening of the skull with temporal lobe white matter ossification of the falx is noted on sagittal T1-weighted images A 56-year-old woman with a 10-year history of myotonic dystrophy type 1 (MD) presented with progressive lower extremity weakness. MRI showed multiple discrete and confluent areas of abnormal signal intensity throughout the subcortical white matter with predominant involvement of the frontal and anterior temporal lobes (figure 1). The confluent white matter lesions in the anterior temporal lobes seen in MD can be radiologically confused with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Unlike CADASIL, MD also displays skull abnormalities (figure 2).1,2 Both syndromes should be considered in patients with confluent temporal lobe lesions on MRI. Laurence A. Donahue, BA, Rajiv Mangla, MD, Per-Lennart Westesson, MD, DDS, PhD, Rochester, NY Disclosure: The authors report no disclosures. Address correspondence and reprint requests to Laurence A. Donahue, Medical Student, University of Rochester School of Medicine and Dentistry, Rochester, NY 14620; [email protected] 1. Miaux Y, Chiras J, Eymard B, et al. Cranial MRI findings in myotonic dystrophy. Neuroradiology 1997;39:166–170. 2. Chabriat H, Joutel