Wildlife on DHI This Is a Snapshot of Some of the Animals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Squamata: Gekkota: Carphodactylidae) from the Pilbara Region of Western Australia
Zootaxa 3010: 20–30 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) A new species of Underwoodisaurus (Squamata: Gekkota: Carphodactylidae) from the Pilbara region of Western Australia PAUL DOUGHTY1,3 & PAUL M. OLIVER2 1Department of Terrestrial Vertebrates, Western Australian Museum, 49 Kew Street, Welshpool, Western Australia 6106, Australia 2Australian Centre for Evolutionary Biology and Biodiversity, University of Adelaide, Adelaide, South Australia, Australia, 5005, and Herpetology Section, South Australian Museum, Adelaide, South Australia 5000, Australia. 3Corresponding author. E-mail: [email protected] Abstract Ongoing surveys and systematic work focused on the Pilbara region in Western Australia have revealed the existence of numerous unrecognized species of reptiles. Here we describe Underwoodisaurus seorsus sp. nov., a new species similar to U. milii, but differing in its relatively plain dorsal and head patterns with only sparsely scattered pale tubercles, a much more gracile build, including longer snout, limbs and digits, smaller and more numerous fine scales on the dorsum, and the enlarged tubercles on the tail tending not to form transverse rows. The new species is known from few specimens and has only been encountered at mid elevations in the Hamersley Ranges, widely separated from the closest populations of U. milii in the northern Goldfields and Shark Bay in Western Australia. Given its rarity and small (potentially relictual) distribution this species may be of conservation concern. Key words: conservation, gecko, Underwoodisaurus milii, relictual distribution Introduction The Pilbara region of Western Australia supports one of the most diverse reptile faunas on the Australian continent (How & Cowan 2006; Powney et al. -

Resolution of the Types and Type Localities of Some Early Nominal Species of the Australian Myobatrachid Frog Genus Pseudophryne Fitzinger, 1843
Zootaxa 4407 (1): 051–064 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4407.1.3 http://zoobank.org/urn:lsid:zoobank.org:pub:99FDA7A2-9C4C-4A7B-99D9-ECC439D06362 Resolution of the types and type localities of some early nominal species of the Australian myobatrachid frog genus Pseudophryne Fitzinger, 1843 GLENN M. SHEA1,2,4 & JODI J.L. ROWLEY2,3 1Sydney School of Veterinary Science B01, Faculty of Science, University of Sydney, NSW 2006, Australia. 2Australian Museum Research Institute, Australian Museum, 1 William St, Sydney, NSW 2010, Australia. 3Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. 4Corresponding author: Glenn M. Shea. E-mail: [email protected] Abstract The types and type localities of Bombinator australis Gray, 1835, Pseudophryne bibronii Günther, 1859, and Phryniscus albifrons Duméril, Bibron & Duméril, 1854, are defined. The nominal type locality for B. australis, Swan River, is con- sidered to be in error. The source of the specimen, Joseph Wright, owned property in the Swan River colony in Western Australia, but later resided in Sydney, the latter locality within the known range of the species. We designate a specimen in the Museum National d’Histoire Naturelle, Paris as lectotype of Pseudophryne bibronii, restricting the type locality of both species to Parramatta, near Sydney, based on the published statements of the collector, François Péron. The holotype of Phryniscus albifrons, a species defined by a painting of a specimen, was likely to have been collected by Jules Verreaux, but the only extant Pseudophryne obtained from Verreaux does not match the type illustration. -

Potential Invasion Risk of Pet Traded Lizards, Snakes, Crocodiles, And
diversity Article Potential Invasion Risk of Pet Traded Lizards, Snakes, Crocodiles, and Tuatara in the EU on the Basis of a Risk Assessment Model (RAM) and Aquatic Species Invasiveness Screening Kit (AS-ISK) OldˇrichKopeck˛ *, Anna Bílková, Veronika Hamatová, Dominika K ˇnazovická, Lucie Konrádová, Barbora Kunzová, Jana Slamˇeníková, OndˇrejSlanina, Tereza Šmídová and Tereza Zemancová Department of Zoology and Fisheries, Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences Prague, Kam˛cká 129, Praha 6 - Suchdol 165 21, Prague, Czech Republic; [email protected] (A.B.); [email protected] (V.H.); [email protected] (D.K.); [email protected] (L.K.); [email protected] (J.S.); [email protected] (B.K.); [email protected] (O.S.); [email protected] (T.S.); [email protected] (T.Z.) * Correspondence: [email protected]; Tel.: +420-22438-2955 !"#!$%&'(! Received: 30 June 2019; Accepted: 9 September 2019; Published: 13 September 2019 !"#$%&' Abstract: Because biological invasions can cause many negative impacts, accurate predictions are necessary for implementing e↵ective restrictions aimed at specific high-risk taxa. The pet trade in recent years became the most important pathway for the introduction of non-indigenous species of reptiles worldwide. Therefore, we decided to determine the most common species of lizards, snakes, and crocodiles traded as pets on the basis of market surveys in the Czech Republic, which is an export hub for ornamental animals in the European Union (EU). Subsequently, the establishment and invasion potential for the entire EU was determined for 308 species using proven risk assessment models (RAM, AS-ISK). Species with high establishment potential (determined by RAM) and at the same time with high potential to significantly harm native ecosystems (determined by AS-ISK) included the snakes Thamnophis sirtalis (Colubridae), Morelia spilota (Pythonidae) and also the lizards Tiliqua scincoides (Scincidae) and Intellagama lesueurii (Agamidae). -

Independent Evolution of Sex Chromosomes in Eublepharid Geckos, a Lineage with Environmental and Genotypic Sex Determination
life Article Independent Evolution of Sex Chromosomes in Eublepharid Geckos, A Lineage with Environmental and Genotypic Sex Determination Eleonora Pensabene , Lukáš Kratochvíl and Michail Rovatsos * Department of Ecology, Faculty of Science, Charles University, 12844 Prague, Czech Republic; [email protected] (E.P.); [email protected] (L.K.) * Correspondence: [email protected] or [email protected] Received: 19 November 2020; Accepted: 7 December 2020; Published: 10 December 2020 Abstract: Geckos demonstrate a remarkable variability in sex determination systems, but our limited knowledge prohibits accurate conclusions on the evolution of sex determination in this group. Eyelid geckos (Eublepharidae) are of particular interest, as they encompass species with both environmental and genotypic sex determination. We identified for the first time the X-specific gene content in the Yucatán banded gecko, Coleonyx elegans, possessing X1X1X2X2/X1X2Y multiple sex chromosomes by comparative genome coverage analysis between sexes. The X-specific gene content of Coleonyx elegans was revealed to be partially homologous to genomic regions linked to the chicken autosomes 1, 6 and 11. A qPCR-based test was applied to validate a subset of X-specific genes by comparing the difference in gene copy numbers between sexes, and to explore the homology of sex chromosomes across eleven eublepharid, two phyllodactylid and one sphaerodactylid species. Homologous sex chromosomes are shared between Coleonyx elegans and Coleonyx mitratus, two species diverged approximately 34 million years ago, but not with other tested species. As far as we know, the X-specific gene content of Coleonyx elegans / Coleonyx mitratus was never involved in the sex chromosomes of other gecko lineages, indicating that the sex chromosomes in this clade of eublepharid geckos evolved independently. -

NSW REPTILE KEEPERS' LICENCE Species Lists 1006
NSW REPTILE KEEPERS’ LICENCE SPECIES LISTS (2006) The taxonomy in this list follows that used in Wilson, S. and Swan, G. A Complete Guide to Reptiles of Australia, Reed 2003. Common names generally follow the same text, when common names were used, or have otherwise been lifted from other publications. As well as reading this species list, you will also need to read the “NSW Reptile Keepers’ Licence Information Sheet 2006.” That document has important information about the different types of reptile keeper licenses. It also lists the criteria you need to demonstrate before applying to upgrade to a higher class of licence. THESE REPTILES CAN ONLY BE HELD UNDER A REPTILE KEEPERS’ LICENCE OF CLASS 1 OR HIGHER Code Scientific Name Common Name Code Scientific Name Common Name Turtles Monitors E2018 Chelodina canni Cann’s Snake-necked Turtle G2263 Varanus acanthurus Spiney-tailed Monitor C2017 Chelodina longicollis Snake-necked Turtle Q2268 Varanus gilleni Pygmy Mulga Monitor G2019 Chelodina oblonga Oblong Turtle G2271 Varanus gouldii Sand Monitor Y2028 Elseya dentata Northern Snapping Turtle M2282 Varanus tristis Black-Headed Monitor K2029 Elseya latisternum Saw-shelled Turtle Y2776 Elusor macrurus Mary River Turtle E2034 Emydura macquarii Murray Short-necked Turtle Skinks T2031 Emydura macquarii dharra Macleay River Turtle A2464 Acritoscincus platynotum Red-throated Skink T2039 Emydura macquarii dharuk Sydney Basin Turtle W2331 Cryptoblepharus virgatus Cream-striped Wall Skink T2002 Emydura macquarii emmotti Emmott’s Short-necked Turtle W2375 -

A LIST of the VERTEBRATES of SOUTH AUSTRALIA
A LIST of the VERTEBRATES of SOUTH AUSTRALIA updates. for Edition 4th Editors See A.C. Robinson K.D. Casperson Biological Survey and Research Heritage and Biodiversity Division Department for Environment and Heritage, South Australia M.N. Hutchinson South Australian Museum Department of Transport, Urban Planning and the Arts, South Australia 2000 i EDITORS A.C. Robinson & K.D. Casperson, Biological Survey and Research, Biological Survey and Research, Heritage and Biodiversity Division, Department for Environment and Heritage. G.P.O. Box 1047, Adelaide, SA, 5001 M.N. Hutchinson, Curator of Reptiles and Amphibians South Australian Museum, Department of Transport, Urban Planning and the Arts. GPO Box 234, Adelaide, SA 5001updates. for CARTOGRAPHY AND DESIGN Biological Survey & Research, Heritage and Biodiversity Division, Department for Environment and Heritage Edition Department for Environment and Heritage 2000 4thISBN 0 7308 5890 1 First Edition (edited by H.J. Aslin) published 1985 Second Edition (edited by C.H.S. Watts) published 1990 Third Edition (edited bySee A.C. Robinson, M.N. Hutchinson, and K.D. Casperson) published 2000 Cover Photograph: Clockwise:- Western Pygmy Possum, Cercartetus concinnus (Photo A. Robinson), Smooth Knob-tailed Gecko, Nephrurus levis (Photo A. Robinson), Painted Frog, Neobatrachus pictus (Photo A. Robinson), Desert Goby, Chlamydogobius eremius (Photo N. Armstrong),Osprey, Pandion haliaetus (Photo A. Robinson) ii _______________________________________________________________________________________ CONTENTS -

Occurrence of the Barking Gecko Underwoodisaurus Milii (Bory 1825) (Gekkonidae) in the Pilbara Region, Western Australia
Journal of the Royal Society of Western Australia, 89:89–90, 2006 Occurrence of the Barking Gecko Underwoodisaurus milii (Bory 1825) (Gekkonidae) in the Pilbara Region, Western Australia M H M Menz1, 2 & P P Cullen1, 3 1ecologia Environment, 1025 Wellington Street West Perth, Western Australia 6005 2Current Address: 8 Princes Street Cottesloe, Western Australia 6011 [email protected] 3Current Address: 9 Madana Place Craigie, Western Australia 6025 Manuscript received June 2005; accepted March 2006 Abstract. To date, a total of five specimens of Underwoodisaurus milii have been collected from the Pilbara Region of Western Australia and lodged at the Western Australian Museum, along with observational records of two other individuals. These records from the Pilbara represent a significant range extension for the species, to the north and east of that currently known for Western Australia. Keywords: Underwoodisaurus milii, Barking Gecko, Thick-tailed Gecko, Pilbara. Introduction Figure 1. Map showing locations for all specimens of U. milii lodged with the WAM, along with locations of some The Barking Gecko Underwoodisaurus milii (Bory observational records. ▲ denotes specimens lodged with the WAM. ✚ denotes Michael Kearney’s record. ★ denotes ecologia 1825) is a species known to occupy a number of habitats, ◆ including woodlands, shrublands, rock outcrops (Storr Environment records from Packsaddle Range. denotes ecologia Environment record from West Angelas. et al. 1990; Wilson & Swan 2003) and under rubbish (Bush et al. 1995), emerging to forage at night (Cogger 2000). Currently the accepted range of this species individual was captured in a rocky gully with Triodia extends across a large part of the southern half of spp. -

Supplementary Methods S1
1 Validation methods for trophic niche models 2 3 To assign links between nodes (species), we used trophic niche-space models (e.g., [1]). 4 Each of these models has two quantile regressions that define the prey-size range a 5 predator of a given size is predicted to consume. Species whose body mass is within the 6 range of a predator’s prey size, as identified by the trophic niche-space model, are predicted 7 to be prey, while those outside the range are predicted not to be eaten. 8 9 The broad taxonomy of a predator helps to predict predation interactions [2]. To optimize 10 our trophic niche-space model, we therefore tested whether including taxonomic class of 11 predators improved the fit of quantile regressions. Using trophic (to identify which species 12 were predators), body mass, and taxonomic data, we fitted and compared five quantile 13 regression models (including a null model) to the GloBI data. In each model, we log10- 14 transformed the dependent variable prey body mass, and included for the independent 15 variables different combinations of log10-transformed predator body mass, predator class, 16 and the interaction between these variables (Supplementary Table S4). We log10- 17 transformed both predator and prey body mass to linearize the relationship between these 18 variables. We fit the five quantile regressions to the upper and lower 5% of prey body mass, 19 and compared model fits using the Bayesian information criterion (BIC). The predator body 20 mass*predator class model fit the 95th quantile data best, whereas the predator body mass 21 + predator class model fit the 5th quantile data marginally better than the aforementioned 22 interaction model (Supplementary Figure S2, Supplementary Table S4). -

Captive Wildlife Regulations, 2021, W-13.12 Reg 5
1 CAPTIVE WILDLIFE, 2021 W-13.12 REG 5 The Captive Wildlife Regulations, 2021 being Chapter W-13.12 Reg 5 (effective June 1, 2021). NOTE: This consolidation is not official. Amendments have been incorporated for convenience of reference and the original statutes and regulations should be consulted for all purposes of interpretation and application of the law. In order to preserve the integrity of the original statutes and regulations, errors that may have appeared are reproduced in this consolidation. 2 W-13.12 REG 5 CAPTIVE WILDLIFE, 2021 Table of Contents PART 1 PART 5 Preliminary Matters Zoo Licences and Travelling Zoo Licences 1 Title 38 Definition for Part 2 Definitions and interpretation 39 CAZA standards 3 Application 40 Requirements – zoo licence or travelling zoo licence PART 2 41 Breeding and release Designations, Prohibitions and Licences PART 6 4 Captive wildlife – designations Wildlife Rehabilitation Licences 5 Prohibition – holding unlisted species in captivity 42 Definitions for Part 6 Prohibition – holding restricted species in captivity 43 Standards for wildlife rehabilitation 7 Captive wildlife licences 44 No property acquired in wildlife held for 8 Licence not required rehabilitation 9 Application for captive wildlife licence 45 Requirements – wildlife rehabilitation licence 10 Renewal 46 Restrictions – wildlife not to be rehabilitated 11 Issuance or renewal of licence on terms and conditions 47 Wildlife rehabilitation practices 12 Licence or renewal term PART 7 Scientific Research Licences 13 Amendment, suspension, -
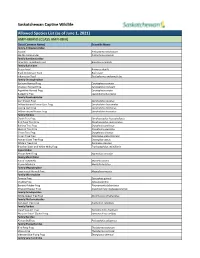
Captive Wildlife Allowed List
Saskatchewan Captive Wildlife Allowed Species List (as of June 1, 2021) AMPHIBIANS (CLASS AMPHIBIA) Class (Common Name) Scientific Name Family Ambystomatidae Axolotl Ambystoma mexicanum Marble Salamander Ambystoma opacum Family Bombinatoridae Oriental Fire-Bellied Toad Bombina orientalis Family Bufonidae Green Toad Anaxyrus debilis Black Indonesian Toad Bufo asper Indonesian Toad Duttaphrynus melanostictus Family Ceratophryidae Surinam Horned Frog Ceratophrys cornuta Chacoan Horned Frog Ceratophrys cranwelli Argentine Horned Frog Ceratophrys ornata Budgett’s Frog Lepidobatrachus laevis Family Dendrobatidae Dart Poison Frog Dendrobates auratus Yellow-banded Poison Dart Frog Dendrobates leucomelas Dyeing Dart Frog Dendrobates tinctorius Yellow-striped Poison Frog Dendrobates truncatus Family Hylidae Clown Tree Frog Dendropsophus leucophyllatus Bird Poop Tree Frog Dendropsophus marmoratus Barking Tree Frog Dryophytes gratiosus Squirrel Tree Frog Dryophytes squirellus Green Tree Frog Dryophytes cinereus Cuban Tree Frog Osteopilus septentrionalis Haitian Giant Tree Frog Osteopilus vastus White’s Tree Frog Ranoidea caerulea Brazilian Black and White Milky Frog Trachycephalus resinifictrix Hyperoliidae African Reed Frog Hyperolius concolor Family Mantellidae Baron’s Mantella Mantella baroni Brown Mantella Mantella betsileo Family Megophryidae Long-nosed Horned Frog Megophrys nasuta Family Microhylidae Tomato Frog Dyscophus guineti Chubby Frog Kaloula pulchra Banded Rubber Frog Phrynomantis bifasciatus Emerald Hopper Frog Scaphiophryne madagascariensis -

Species Richness in Time and Space: a Phylogenetic and Geographic Perspective
Species Richness in Time and Space: a Phylogenetic and Geographic Perspective by Pascal Olivier Title A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Ecology and Evolutionary Biology) in The University of Michigan 2018 Doctoral Committee: Assistant Professor and Assistant Curator Daniel Rabosky, Chair Associate Professor Johannes Foufopoulos Professor L. Lacey Knowles Assistant Professor Stephen A. Smith Pascal O Title [email protected] ORCID iD: 0000-0002-6316-0736 c Pascal O Title 2018 DEDICATION To Judge Julius Title, for always encouraging me to be inquisitive. ii ACKNOWLEDGEMENTS The research presented in this dissertation has been supported by a number of research grants from the University of Michigan and from academic societies. I thank the Society of Systematic Biologists, the Society for the Study of Evolution, and the Herpetologists League for supporting my work. I am also extremely grateful to the Rackham Graduate School, the University of Michigan Museum of Zoology C.F. Walker and Hinsdale scholarships, as well as to the Department of Ecology and Evolutionary Biology Block grants, for generously providing support throughout my PhD. Much of this research was also made possible by a Rackham Predoctoral Fellowship, and by a fellowship from the Michigan Institute for Computational Discovery and Engineering. First and foremost, I would like to thank my advisor, Dr. Dan Rabosky, for taking me on as one of his first graduate students. I have learned a tremendous amount under his guidance, and conducting research with him has been both exhilarating and inspiring. I am also grateful for his friendship and company, both in Ann Arbor and especially in the field, which have produced experiences that I will never forget. -

Small Vertebrate Colonisers of Mine Site Rehabilitated Waste Dumps in the Goldfields of Western Australia
Mine Closure 2006 ― Andy Fourie and Mark Tibbett (eds) © 2006 Australian Centre for Geomechanics, Perth, ISBN 0-9756756-6-4 https://papers.acg.uwa.edu.au/p/605_24_Thompson/ Small Vertebrate Colonisers of Mine Site Rehabilitated Waste Dumps in the Goldfields of Western Australia G.G. Thompson Centre for Ecosystem Management, Edith Cowan University, Australia S.A. Thompson ATA Environmental, Australia 1 INTRODUCTION Mine site waste dumps pass through various stages as they progress towards the development of mature ecosystems. The ultimate ecosystem on a rehabilitated waste dump is largely determined by the soils and vegetation, and connections with the adjacent habitats that enable invertebrates and vertebrates to move into this area. For some mine sites, the primary objective is to create near-natural, self-sustaining functional ecosystems, others settle for lesser outcomes. To achieve a near-natural, self-sustaining, functional ecosystem is not easy and a lofty objective because of the difficulty in creating the weathered topography and soils of the region, and creating vegetation assemblages of natural ecosystems. Most often waste dumps are huge structures that rise above the existing soil profile, are filled with mining waste in the sequence that it is extracted from the mine, and have a top soil capping that is ripped to reduce erosion and maximise water penetration rather than running off. In the Western Australian Goldfields, mining waste can contain pyrite, which when exposed to water and oxygen increases soil acidity, can contain hypersaline water, may contain concentrations of toxic chemicals or may be hard rock, all of which provide challenges for mine site rehabilitation planners and extra difficulties in achieving near-natural, functional ecosystems as final outcomes for rehabilitated areas.