NOR03001.Pdf(PDF, 827
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NEWSLETTER NUMBER 84 JUNE 2006 New Zealand Botanical Society
NEW ZEALAND BOTANICAL SOCIETY NEWSLETTER NUMBER 84 JUNE 2006 New Zealand Botanical Society President: Anthony Wright Secretary/Treasurer: Ewen Cameron Committee: Bruce Clarkson, Colin Webb, Carol West Address: c/- Canterbury Museum Rolleston Avenue CHRISTCHURCH 8001 Subscriptions The 2006 ordinary and institutional subscriptions are $25 (reduced to $18 if paid by the due date on the subscription invoice). The 2006 student subscription, available to full-time students, is $9 (reduced to $7 if paid by the due date on the subscription invoice). Back issues of the Newsletter are available at $2.50 each from Number 1 (August 1985) to Number 46 (December 1996), $3.00 each from Number 47 (March 1997) to Number 50 (December 1997), and $3.75 each from Number 51 (March 1998) onwards. Since 1986 the Newsletter has appeared quarterly in March, June, September and December. New subscriptions are always welcome and these, together with back issue orders, should be sent to the Secretary/Treasurer (address above). Subscriptions are due by 28th February each year for that calendar year. Existing subscribers are sent an invoice with the December Newsletter for the next years subscription which offers a reduction if this is paid by the due date. If you are in arrears with your subscription a reminder notice comes attached to each issue of the Newsletter. Deadline for next issue The deadline for the September 2006 issue is 25 August 2006 Please post contributions to: Joy Talbot 17 Ford Road Christchurch 8002 Send email contributions to [email protected] or [email protected]. Files are preferably in MS Word (Word XP or earlier) or saved as RTF or ASCII. -

Recent Changes in the Names of New Zealand Tree and Shrub Species
-- -- - Recent changes in the names of New Zealand tree and shrub species - Since the publication of 'Flora of New Zealand' Volume 1 (A- iii) Podocarpus dacydioides Dacrycarpus ducydioides lan 1961),covering indigenous ferns, conifers and dicots, there (iii)Podocarpus ferrugzneus Prumnopitys ferruginea have been major advances in taxonomic research and the clas- Podocarpus spicatus Prumnopitys taxijolia sification of many plant groups revised accordingly. Most of (iv1 Dacrydium cupressinum (unchanged) these changes have been summarised in the Nomina Nova (v)Dacrydium bidwillii Halocarpus bidwillii series published in the New Zealand Journal of Botany (Edgar Dacrydium bijorme Halocarpus bijormis 1971, Edgar and Connor 1978, 1983) and are included in re- Dacrydium kirkii Halocarpus kirkii cent books on New Zealand plants ie.g. Eagle 1982, Wilson (vi)Dacydium colensoi Lagarostrobos colensoi 1982). A number of these name changes affect important (vii)Dacrydium intermediurn Lepidothamnus intermedius forest plants and as several of these new names are now start- Dacrydium laxijolium Lepidotbamnus laxijolius ing to appear in the scientific literature, a list of changes af- (viii)Phyllocladus trichomanoidi~(unchanged) fecting tree and shrub taxa are given here. As a large number Phyllocladus glaucus (unchanged) of the readers of New Zealand Forestry are likely to use Poole Phyllocladus alpinus Phyllocladus aspleniijolius and Adams' "Trees and Shrubs of New Zealand" as their var. alpinus* * main reference for New Zealand forest plants, all the name changes are related to the fourth impression of this book. * It has been suggested that the Colenso name P, cunnin- it is important to realise that not all botanists necessarily ghamii (1884)should take precedence over the later (18891 ark agree with one particular name and you are not obliged to use name (P. -
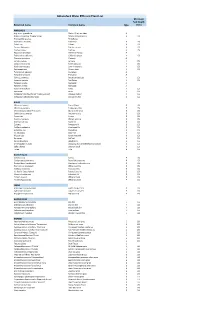
Abbotsford Water Efficient Plant List Minimum Soil Depth Botanical Name Common Name Type (Mm)
Abbotsford Water Efficient Plant List Minimum Soil Depth Botanical name Common name type (mm) ANNUALS Argemone grandifolia Statice/Sea Lavendar A Begonia x hybrida 'Dragon Wings' Dragon Wing begonia A 150 Bracteantha species Strawflower Calendula officinalis Calendula A 150 Coleus ssp. Coleus A 150 Cosmos bipinnatus Garden cosmos A 150 Cuphea llavea Cuphea A 150 Dyssocua tenuiloba Dahlberg Daisey A Eschscholzia californica California poppy A 150 Gazania spendens Gazania A Lantana camara Lantana A 150 Lobularia maritima Sweet alyssum A 150 Nigella damascena Love-in-the-mist A 150 Oesteopermum African daisy A 150 Pelargonium species Geranium A Portulaca oleracea Portulaca A Salvia guaranitica Anise-scented sage A 150 Scaevola aemula Fan flower A 150 Targetes erecta Marogold A Targetes erecta Marogold A Viola x wittrockiana Pansy A 150 Zinnia ssp. Zinnia A 150 Verbascum bombyciferum 'Arctic Summer' Broussa mullein A 150 Verbascum phlomoides 'Spica' Orange mullein A 150 BULBS Allium christophii Star of Persia B 150 Allium karataviense Turkestan onion B 150 Chionodoxa forbesii 'Pink Giant' Glory of the snow B 150 Colchicum autumnale Autumn crocus B 150 Crocus ssp. Crocus B 150 Eranthis hyemalis Winter aconite B 150 Erythronium ssp. Fawn lily B 150 Eucomis Pineapple lily B 150 Fritillaria meleagris Checkered lily B 150 Galanthus ssp. Snowdrop B 150 Iris reticulata Dwarf iris B 150 Muscari ssp. Grape hyacinth B 150 Narcissus Daffodil B 150 Nerine bowdenii Bowden lily B 150 Ornithogalum nutans Drooping Star of Bethlehem/Silverbells B 150 Scilla -

Brachyglottis Heeft Zomer- Tweede Tong of Lip (Zoals Aanwezig Bij Onder Andere De Bloemen Van Orchideeën)
BOOMKWEKERIJ In de winter opvallend met het viltige grijze blad en in de zomer vrolijk kleurend door een overdaad aan gele ‘mar- grieten’. Brachyglottis is een plant voor twaalf maanden tuinplezier die het op een beschutte standplaats prima naar de zin heeft. Tekst: Emiel van den Berg Foto: www.visionspictures.com lle Brachyglottis-soorten behoorden vroeger tot het geslacht Senecio, een Avan de grootste plantengeslachten van het plantenrijk. Sinds de jaren zeventig van de vorige eeuw is het geslacht Brachyglottis geïn- troduceerd en zijn hier ruim 25 soorten van voorheen Senecio in ondergebracht, soorten die van oorsprong voornamelijk in Nieuw-Zee- land en Tasmanië groeien. Brachyglottis greyi De wetenschappelijke naam Brachyglottis is opgebouwd uit de Griekse woorden brachus en glossa. Het eerste woord betekent kort, het Brachyglottis heeft zomer- tweede tong of lip (zoals aanwezig bij onder andere de bloemen van orchideeën). In dit geval verwijst het woord naar de korte lintbloe- en winterkwaliteiten men. Kenmerkend voor de bloem van Brac- hyglottis is dat wat één bloem lijkt, in werkelijk- zomergevoel. Door na de bloei te snoeien – in lijkbaar is met het blad van B. ‘Sunshine’ maar heid is opgebouwd uit een groot aantal dicht landen met een warmer klimaat dan in Neder- de bladrand is gegolfd. Opvallend zijn de wit- opeenstaande buis- of lintbloemen. land wordt hij vaak als losse haagplant gebruikt behaarde jonge scheuten, bladstelen en bloem- Het geslacht Brachyglottis behoort tot de fami- – blijft de plant vitaal en de habitus compact. stengels. In delen van Engeland en Ierland is lie van de samengesteldbloemigen, de Compo- Bij strenge winters kan echter wel vorstscha- B. -

Co-Extinction of Mutualistic Species – an Analysis of Ornithophilous Angiosperms in New Zealand
DEPARTMENT OF BIOLOGICAL AND ENVIRONMENTAL SCIENCES CO-EXTINCTION OF MUTUALISTIC SPECIES An analysis of ornithophilous angiosperms in New Zealand Sandra Palmqvist Degree project for Master of Science (120 hec) with a major in Environmental Science ES2500 Examination Course in Environmental Science, 30 hec Second cycle Semester/year: Spring 2021 Supervisor: Søren Faurby - Department of Biological & Environmental Sciences Examiner: Johan Uddling - Department of Biological & Environmental Sciences “Tui. Adult feeding on flax nectar, showing pollen rubbing onto forehead. Dunedin, December 2008. Image © Craig McKenzie by Craig McKenzie.” http://nzbirdsonline.org.nz/sites/all/files/1200543Tui2.jpg Table of Contents Abstract: Co-extinction of mutualistic species – An analysis of ornithophilous angiosperms in New Zealand ..................................................................................................... 1 Populärvetenskaplig sammanfattning: Samutrotning av mutualistiska arter – En analys av fågelpollinerade angiospermer i New Zealand ................................................................... 3 1. Introduction ............................................................................................................................... 5 2. Material and methods ............................................................................................................... 7 2.1 List of plant species, flower colours and conservation status ....................................... 7 2.1.1 Flower Colours ............................................................................................................. -

Lighting up with Foliage
LightingLighting UpUp withwith FoliageFoliage Variegated and silver foliage can be the foundation for a garden that shines all season long. BY HEATHER PRINCE HOUGH NOT considered col- Canada, and former director of horti- ors in the same way as, say, red Resembling forget-me-nots, the flowers of culture at the Toronto Botanical Gar- T and blue, white and silver can Brunnera macrophylla ‘Jack Frost’ bloom for den. “There’s a real beauty in whites be equally powerful in the garden. They weeks as the silver-washed, heart-shaped and silvers. It’s subtle, but impactful, can evoke a feeling of serenity, provide foliage shimmers in the shade. This tidy, cool and calming. It can really create an welcome brightness in shade, or add a clump-forming perennial thrives in dry shade atmosphere.” sense of sophistication and elegance. “It beneath trees. Its foliage, which doubles Of course, there are myriad white flow- doesn’t have to be bold to be beautiful,” as a sophisticated addition to cut flower ers available from beloved heirlooms to says Paul Zammit, a professor of horti- arrangements, looks good well into fall. hot new cultivars, but for lasting power GRAHAM RICE, GARDENPHOTOS.COM culture at Niagara College in Ontario, over the growing season, use foliage to July / August 2021 11 provide structure, texture, and color. This is especially important if you’re creating an evening or moon garden to be enjoyed in low light. When silver foliage is paired with white flowers, you can layer in even more nighttime enjoyment. THE MANY SHADES OF WHITE AND SILVER “White” foliage in plants is never white in the way that flowers can be. -

NORTHLAND Acknowledgements
PLANT ME INSTEAD! NORTHLAND Acknowledgements Thank you to the following people and organisations who helped with the production of this booklet: Northland Regional Council staff, and Department of Conservation staff, Northland, for participation, input and advice; John Barkla, Jeremy Rolfe, Trevor James, John Clayton, Peter de Lange, John Smith-Dodsworth, John Liddle (Liddle Wonder Nurseries), Clayson Howell, Geoff Bryant, Sara Brill, Andrew Townsend and others who provided photos; Sonia Frimmel (What’s the Story) for design and layout. While all non-native alternatives have been screened against several databases to ensure they are not considered weedy, predicting future behaviour is not an exact science! The only way to be 100% sure is to use ecosourced native species. Published by: Weedbusters © 2011 ISBN: 978-0-9582844-9-3 Get rid of a weed, plant me instead! Many of the weedy species that are invading and damaging our natural areas are ornamental plants that have ‘jumped the fence’ from gardens and gone wild. It costs councils, government departments and private landowners millions of dollars, and volunteers and community groups thousands of unpaid hours, to control these weeds every year. This Plant Me Instead booklet profiles the environmental weeds of greatest concern to those in your region who work and volunteer in local parks and reserves, national parks, bush remnants, wetlands and coastal areas. Suggestions are given for locally-sold non-weedy species, both native and non- native, that can be used to replace these weeds in your garden. We hope that this booklet gives you some ideas on what you can do in your own backyard to help protect New Zealand’s precious environment. -

Deer Resistant Plant List
Victoria Master Gardener Association 1 Deer Resistant Plant List The following information has been drawn from a variety of sources including: the Island Specialty Nursery Catalogue, 1999; The Island Grower, August/September, 1994; Cannor Nursery; The Home, Yard & Garden Pest Newsletter, University of Illinois Extension, 1997; Sunset Western Gardener; “Living with Wildlife in the Pacific Northwest” by Russell Link (University of Washington Press) and Dinter Nursery. General comments about deer-proof plants: • The most reliably deer-proof plants are those with acrid sap like Euphorbias; strongly scented foliage like lavender, sages and oregano; dry-loving plants like Cistus and Halimium; fuzzy leafed plants like Stachys and Pulmonaria; also most plants with grey/silver foliage such as dusty millers. • Deer use their sense of smell to detect predators. Strongly scented plants may “confuse” deer’s sense of smell, which makes them uneasy. Plants with strongly scented foliage may therefore act to deter deer from an area. Consider several plants with varying strong scents planted around the deer’s usual pathway to your property, and as a perimeter around other plants as a possible protective/deterrent strategy. Good candidates include santolina, helichrysum italicum (curry plant), Ruta graveolens (Jackman’s rue), Lantana, and very aromatic herbs like thyme and oregano. • Plants not browsed for years can fall prey whenever food is in very short supply and the deer are desperate. Deer will “remember” these plants as edible and may start browsing them regularly in future years. Some gardeners have successfully planted a perimeter garden of “deer fodder” to provide food so the garden plants are left alone. -

SPRING 2020 Mail Order Catalog Cistus Nursery
SPRING 2020 Mail Order Catalog Cistus Nursery 22711 NW Gillihan Road Sauvie Island, OR 97231 503.621.2233 phone Spring 2020 Mail Order Catalog order by phone 9 - 5 pst, visit 10am - 5pm, email: [email protected] www.cistus.com 2 USDA zone: Aeschynanthus buxifolius $16 Gesneriaceae Antirrhinum braun-blanquetii [red-leaved] $14 Arctostaphylos 'Pebbles' $15 Ericaceae Arctostaphylos mendocinoensis SBH 12150b $16 Ericaceae Arctostaphylos mewukka SBH/GPP 12133 $15 Arctostaphylos nevadensis x SBH 12189 $15 Ericaceae Arctostaphylos pumila [grey selection] $15 Arctostaphylos stanfordiana SBH 9834 $16 Ericaceae artemisia mollenerii $11 Asteraceae Aspidistra attenuata BSWJ 377 attenuate cast iron plant $16 Asparagaceae Aucuba sp. [Willis H.] $15 Garryaceae Spring 2020 Mail Order Catalog 3 berberis 'Lime Blow' $14 Berberidaceae Bletilla striata 'Big Bob' ground orchid $16 orchidaceae Ceanothus x SBH 12334a $14 Chamaecereus sylvestri peanut cactus $12 Cactaceae Chasmanthe floribunda 'variegata' $11 Iridaceae Choisya dumosa $15 Rutaceae Cistus x 'Christopher Gable' $12 Cistaceae Colletia ulcinia [from Western Hills 10-16-18] $16 Rhamnaceae Cotoneaster dammeri 'Coral Beauty' $12 Rosaceae Dichelostemma capitatum large form $12 Asparagaceae Fuchsia microphylla 'Silver Lining' $14 Onagraceae Spring 2020 Mail Order Catalog 4 garrya fremontii (flavescens influenced) SBH 10037 $15 Ericaceae Gasteria baylissiana 'Variegata' $11 Graptopetalum pentandrum $9 Haworthia tessellata - UK Coll. cl 1 $9 Xanthorrhoeaceae Hemiboea subcapitata $14 Gesneriaceae Hesperoyucca -

New Zealand Plants in Australian Gardens Stuart Read (Updated 27/5/2018)
New Zealand Plants in Australian Gardens Stuart Read (updated 27/5/2018) Abstract: (11.6.2013): Raised in a large New Zealand garden full of native trees, plant lover Stuart Read was perhaps hard-wired to notice kiwi plants in Australian gardens. Over time he's pieced together a pattern of waves of fashion in their planting and popularity, reflecting scientific and horticultural expansionism, commercial and familial networks and connections across the Tasman. Stuart will examine a range of NZ plants found in old and younger Australian gardens, try to tease out some of the means by which they got here and why they remain popular. No cabbage, This constellation of asterisks Slaps and rustles Its tough tatters In the brisk breeze; Whispers of times past And ancient histories (Barbara Mitcalfe’s poem, ‘Ti Kouka’ (cabbage tree) catches well the distinctive skyline profile of this ubiquitous New Zealand export (in Simpson, 2000, 213) Introduction / overview New Zealand gardens have been introduced to and cultivated in Australian gardens from early in their ‘discovery’, trade and exchanges between the two colonies. Australian and other explorers, botanists, nurserymen, New Zealand settlers and others searched New Zealand’s coasts and bush, bringing plants into cultivation, export and commerce from early in the settlement’s colonization. New Zealand plants have had their ‘vogue’ periods, including as: A) - Economic plants (various timbers, kauri gum for shellacs and jewellery; flax for fibre, rope, cloth; greens for scurvy; poroporo for the contraceptive ‘the pill’); B) - Exotic ornamental imports into Australian gardens and beyond to English and European conservatories (and some warmer, southern) gardens and parks; C) - Depicted or carved as subjects of botanical and other artworks, commercial commodities. -

Lincoln University Digital Dissertation
Lincoln University Digital Dissertation Copyright Statement The digital copy of this dissertation is protected by the Copyright Act 1994 (New Zealand). This dissertation may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the dissertation and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the dissertation. Insect flower visitors in native plantings within the arable landscape of the Canterbury Plains A Dissertation submitted in partial fulfilment of the requirements for the Degree of Masters in Agricultural Science at Lincoln University by Franziska Gabriela Schmidlin Lincoln University 2018 Leiproctus fulvescens on Veronica salicifolia Abstract of a Dissertation submitted in partial fulfilment of the requirements for the Degree of Masters in Agricultural Science. Abstract Insect flower visitors in native plantings within the arable landscape of the Canterbury Plains by Franziska Gabriela Schmidlin This thesis investigates the value of native plantings for pollination services within the arable landscape of Mid-Canterbury, New Zealand. The vegetation of the Canterbury Plains is among the most heavily modified landscapes in New Zealand with almost all original native vegetation replaced by intensive dairy and arable farming. Arable farmers often grow a variety of vegetable or herbage seed crops that depend on insect pollination. These include carrot, radish, onion, brassicas, and white and red clover. Intensive crop farming on the Canterbury Plains can therefore be highly dependent on a good provision of insect pollinators to maintain economically viable yields. -
Coastal Butterflies and Moths of Wellington and South Wairarapa
wgnco-52656 COASTAL BUTTERFLIES AND MOTHS OF WELLINGTON AND SOUTH WAIRARAPA Brian H Patrick Otago Museum Box 6202 Dunedin 1. INTRODUCTION 1.1 The Setting It has long been recognised that the wild coastline of the southern North Island is home to a rich array of native insects and plants. Rich not just because of an abundance of species but also because an upland element – species more typical of alpine and montane areas of the South Island is present. This is an exciting coastline for the naturalist with high cliffs and steep slopes clothed in low shrubs, herbs and grasses, scree-like gravel slopes and beaches, rocky headlands and shrubby hillsides. Contrasting bone-dry divaricating shrublands on one hand and lush sedgelands and herbfields sheltering in low forest on the other. The landscape is high in natural values. In many ways this coast looks and feels like part of the upland South Island, and in fact many of its species are either the same or closely related to such species in upland habitats on the other side of Cook Strait. Several high profile insects are present on this coastline, although neither is widespread there. These include the following; Huttons speargrass weevil Lyperobius huttoni a large-bodied species typical of the eastern South Island high country Myers black cicada Maoricicada myersi a southern Wellington endemic species The former is an upland species here at its northern limit – possibly a relict distribution (Fleming 1971). It is more widely known from the eastern South Island south to the Waitaki River, while the latter has most of its close relatives singing from rocky vantage points on the many peaks of the South Island.