BCAT1 Expression Associates with Ovarian Cancer Progression: Possible Implications in Altered Disease Metabolism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ovulation-Selective Genes: the Generation and Characterization of an Ovulatory-Selective Cdna Library
531 Ovulation-selective genes: the generation and characterization of an ovulatory-selective cDNA library A Hourvitz1,2*, E Gershon2*, J D Hennebold1, S Elizur2, E Maman2, C Brendle1, E Y Adashi1 and N Dekel2 1Division of Reproductive Sciences, Department of Obstetrics and Gynecology, University of Utah Health Sciences Center, Salt Lake City, Utah 84132, USA 2Department of Biological Regulation, Weizmann Institute of Science, Rehovot, Israel (Requests for offprints should be addressed to N Dekel; Email: [email protected]) *(A Hourvitz and E Gershon contributed equally to this paper) (J D Hennebold is now at Division of Reproductive Sciences, Oregon National Primate Research Center, Oregon Health and Science University, Beaverton, Oregon 97006, USA) Abstract Ovulation-selective/specific genes, that is, genes prefer- (FAE-1) homolog, found to be localized to the inner entially or exclusively expressed during the ovulatory periantral granulosa and to the cumulus granulosa cells of process, have been the subject of growing interest. We antral follicles. The FAE-1 gene is a -ketoacyl-CoA report herein studies on the use of suppression subtractive synthase belonging to the fatty acid elongase (ELO) hybridization (SSH) to construct a ‘forward’ ovulation- family, which catalyzes the initial step of very long-chain selective/specific cDNA library. In toto, 485 clones were fatty acid synthesis. All in all, the present study accom- sequenced and analyzed for homology to known genes plished systematic identification of those hormonally with the basic local alignment tool (BLAST). Of those, regulated genes that are expressed in the ovary in an 252 were determined to be nonredundant. -

Isocitrate Dehydrogenase 1 (NADP+) (I5036)
Isocitrate Dehydrogenase 1 (NADP+), human recombinant, expressed in Escherichia coli Catalog Number I5036 Storage Temperature –20 °C CAS RN 9028-48-2 IDH1 and IDH2 have frequent genetic alterations in EC 1.1.1.42 acute myeloid leukemia4 and better understanding of Systematic name: Isocitrate:NADP+ oxidoreductase these mutations may lead to an improvement of (decarboxylating) individual cancer risk assessment.6 In addition other studies have shown loss of IDH1 in bladder cancer Synonyms: IDH1, cytosolic NADP(+)-dependent patients during tumor development suggesting this may isocitrate dehydrogenase, isocitrate:NADP+ be involved in tumor progression and metastasis.7 oxidoreductase (decarboxylating), Isocitric Dehydrogenase, ICD1, PICD, IDPC, ICDC, This product is lyophilized from a solution containing oxalosuccinate decarboxylase Tris-HCl, pH 8.0, with trehalose, ammonium sulfate, and DTT. Product Description Isocitrate dehydrogenase (NADP+) [EC 1.1.1.42] is a Purity: ³90% (SDS-PAGE) Krebs cycle enzyme, which converts isocitrate to a-ketoglutarate. The flow of isocitrate through the Specific activity: ³80 units/mg protein glyoxylate bypass is regulated by phosphorylation of isocitrate dehydrogenase, which competes for a Unit definition: 1 unit corresponds to the amount of 1 common substrate (isocitrate) with isocitrate lyase. enzyme, which converts 1 mmole of DL-isocitrate to The activity of the enzyme is dependent on the a-ketoglutarate per minute at pH 7.4 and 37 °C (NADP formation of a magnesium or manganese-isocitrate as cofactor). The activity is measured by observing the 2 complex. reduction of NADP to NADPH at 340 nm in the 7 presence of 4 mM DL-isocitrate and 2 mM MnSO4. -

NRF2 and the Ambiguous Consequences of Its Activation During Initiation and the Subsequent Stages of Tumourigenesis
cancers Review NRF2 and the Ambiguous Consequences of Its Activation during Initiation and the Subsequent Stages of Tumourigenesis Holly Robertson 1,2, Albena T. Dinkova-Kostova 1 and John D. Hayes 1,* 1 Jacqui Wood Cancer Centre, Division of Cellular Medicine, Ninewells Hospital and Medical School, University of Dundee, Dundee DD1 9SY, Scotland, UK; [email protected] (H.R.); [email protected] (A.T.D.-K.) 2 Wellcome Trust Sanger Institute, Wellcome Genome Campus, Cambridge CB10 1SA, UK * Correspondence: [email protected]; Tel.: +44-(0)-1382-383182 Received: 30 October 2020; Accepted: 27 November 2020; Published: 2 December 2020 Simple Summary: Transcription factor NRF2 controls expression of antioxidant and detoxification genes. Normally, the activity of NRF2 is tightly controlled in the cell, and is continuously adjusted to ensure that cells are protected against endogenous chemicals and environmental agents that perturb the intracellular antioxidant/pro-oxidant balance (i.e., redox) that must be maintained for them to grow and survive in an appropriate manner. This tight control of NRF2 is achieved by a repressor protein called KEAP1 that perpetually targets NRF2 protein for degradation under normal conditions, but is unable to do so when challenged with oxidants or thiol-reactive chemicals. In the context of cancer, it is well known that drugs that stimulate short-term and reversible activation of NRF2 can provide protection for a limited period against exposure to chemicals that cause cancer. However, it is also becoming widely recognised that permanent hyper-activation of NRF2 resulting from somatic mutations in the gene that encodes NRF2, or in genes associated with its degradation, is frequently observed in certain cancers and associated with poor outcome. -

Development of Potent and Selective Inhibitors of Aldo−Keto Reductase
Article pubs.acs.org/jmc Development of Potent and Selective Inhibitors of Aldo−Keto Reductase 1C3 (Type 5 17β-Hydroxysteroid Dehydrogenase) Based on N-Phenyl-Aminobenzoates and Their Structure−Activity Relationships † § ‡ § † † † ‡ Adegoke O. Adeniji, , Barry M. Twenter, , Michael C. Byrns, Yi Jin, Mo Chen, Jeffrey D. Winkler,*, † and Trevor M. Penning*, † Department of Pharmacology and Center of Excellence in Environmental Toxicology, Perelman School of Medicine, University of Pennsylvania, 130C John Morgan Building, 3620 Hamilton Walk, Philadelphia, Pennsylvania 19104-6084, United States ‡ Department of Chemistry, University of Pennsylvania, 231 South 34th Street, Philadelphia, Pennsylvania 19104, United States *S Supporting Information ABSTRACT: Aldo−keto reductase 1C3 (AKR1C3; type 5 17β-hydroxy- steroid dehydrogenase) is overexpressed in castration resistant prostate cancer (CRPC) and is implicated in the intratumoral biosynthesis of testosterone and 5α-dihydrotestosterone. Selective AKR1C3 inhibitors are required because compounds should not inhibit the highly related AKR1C1 and AKR1C2 isoforms which are involved in the inactivation of 5α-dihydrotestosterone. NSAIDs, N-phenylanthranilates in particular, are potent but nonselective AKR1C3 inhibitors. Using flufenamic acid, 2-{[3-(trifluoromethyl)phenyl]amino}- benzoic acid, as lead compound, five classes of structural analogues were synthesized and evaluated for AKR1C3 inhibitory potency and selectivity. Structure−activity relationship (SAR) studies revealed that a meta-carboxylic acid group relative to the amine conferred pronounced AKR1C3 selectivity without loss of potency, while electron withdrawing groups on the phenylamino B-ring were optimal for AKR1C3 inhibition. Lead compounds did not inhibit COX-1 or COX-2 but blocked the AKR1C3 mediated production of testosterone in LNCaP-AKR1C3 cells. These compounds offer promising leads toward new therapeutics for CRPC. -

Energy Metabolism in IDH1 Wild-Type and IDH1-Mutated Glioblastoma Stem Cells: a Novel Target for Therapy?
cells Review Energy Metabolism in IDH1 Wild-Type and IDH1-Mutated Glioblastoma Stem Cells: A Novel Target for Therapy? Cornelis J.F. van Noorden 1,2,*, Vashendriya V.V. Hira 1, Amber J. van Dijck 2 , Metka Novak 1, Barbara Breznik 1 and Remco J. Molenaar 1,3 1 Department of Genetic Toxicology and Cancer Biology, National Institute of Biology, VeˇcnaPot 111, 1000 Ljubljana, Slovenia; [email protected] (V.V.V.H.); [email protected] (M.N.); [email protected] (B.B.); [email protected] (R.J.M.) 2 Department of Medical Biology, Amsterdam UMC Location Academic Medical Center, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] 3 Department of Medical Oncology, Amsterdam UMC Location Academic Medical Center, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands * Correspondence: [email protected]; Tel.: +31-638-639-561 Abstract: Cancer is a redox disease. Low levels of reactive oxygen species (ROS) are beneficial for cells and have anti-cancer effects. ROS are produced in the mitochondria during ATP production by oxidative phosphorylation (OXPHOS). In the present review, we describe ATP production in primary brain tumors, glioblastoma, in relation to ROS production. Differentiated glioblastoma cells mainly use glycolysis for ATP production (aerobic glycolysis) without ROS production, whereas glioblastoma stem cells (GSCs) in hypoxic periarteriolar niches use OXPHOS for ATP and ROS production, which is modest because of the hypoxia and quiescence of GSCs. In a significant proportion of glioblastoma, Citation: van Noorden, C.J.F.; Hira, isocitrate dehydrogenase 1 (IDH1) is mutated, causing metabolic rewiring, and all cancer cells use V.V.V.; van Dijck, A.J.; Novak, M.; OXPHOS for ATP and ROS production. -

Methylation-Dependent Tissue Factor Suppression Contributes to the Reduced Malignancy of IDH1 Mutant Gliomas
Author Manuscript Published OnlineFirst on September 28, 2018; DOI: 10.1158/1078-0432.CCR-18-1222 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Unruh et al. Methylation-dependent Tissue Factor suppression contributes to the reduced malignancy of IDH1 mutant gliomas 1 1 2 1 1 Dusten Unruh, Snezana Mirkov, Brian Wray, Michael Drumm, Jonathan Lamano, Yuping D Li,1 Qazi F. Haider,1 Rodrigo Javier,1 Kathleen McCortney,1 Amanda Saratsis,1 Denise M. Scholtens,3 Jann N. Sarkaria,4 C. David James,1 Craig Horbinski1,5 1Department of Neurological Surgery, Northwestern University, Chicago, IL, 60611, USA 2Department of Biochemistry and Molecular Genetics, Northwestern University, Chicago, IL, 60611, USA 3Department of Preventive Medicine, Northwestern University, Chicago, IL, 60611, USA 4Department of Radiation Oncology, Mayo Clinic, Rochester, MN, 55905, USA 5Department of Pathology, Northwestern University, Chicago, IL, 60611, USA RUNNING TITLE Tissue Factor in IDH1mut and IDH1wt gliomas KEYWORDS Tissue Factor; glioma; isocitrate dehydrogenase; beta-catenin; protease-activated receptor FINANCIAL SUPPORT This work was supported by NIH grants K08CA155764 and R01NS102669 (C.H.). D.U. was supported by NIH grants T32CA070085 and F32CA216996. C.H., D.M.S., and C.D.J. were also supported by the Northwestern SPORE in Brain Cancer P50CA221747. The Mayo Clinic Brain Tumor Patient-Derived Xenograft National Resource is supported by Mayo Clinic, the Mayo SPORE in Brain Cancer P50CA108961 and NIH grant R24NS92940. Histology and fluorescent 1 Downloaded from clincancerres.aacrjournals.org on September 23, 2021. © 2018 American Association for Cancer Research. Author Manuscript Published OnlineFirst on September 28, 2018; DOI: 10.1158/1078-0432.CCR-18-1222 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. -
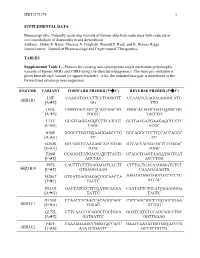
Data Supplement
JPET#173179 1 SUPPLEMENTAL DATA Manuscript title: Naturally occurring variants of human aldo-keto reductases with reduced in vitro metabolism of daunorubicin and doxorubicin Authors: Onkar S. Bains, Thomas A. Grigliatti, Ronald E. Reid, and K. Wayne Riggs Journal name: Journal of Pharmacology and Experimental Therapeutics TABLES Supplemental Table 1—Primers for creating non-synonymous single nucleotide polymorphic variants of human AKRs and CBR4 using site-directed mutagenesis. The base pair mutation is given beneath each variant [in square brackets]. Also, the mutated base pair is underlined in the forward and reverse primer sequences. ENZYME VARIANT FORWARD PRIMER (5'3') REVERSE PRIMER (5'3') I15F CAAGATGCCCTTCCTGGGGTT CCAACCCCAGGAAGGGCATC AKR1B1 [AT] GG TTG H42L CGGGTACCGCCTCATCGACTG GGGCACAGTCGATGAGGCGG [AT] TGCCC TACCCG L73V GCGTGAGGAGGTCTTCATCGT GCTGACGATGAAGACCTCCTC [CG] CAGC ACGC K90E GGGCCTGGTGGAAGGAGCCTG GGCAGGCTCCTTCCACCAGGC [AG] CC CC G204S GCCAGTCCAAAAGCATCGTGG GTCACCACGATGCTTTTGGAC [GA] TGAC TGGC T288I CCAGGATATGACCATCTTACTC GTAGCTGAGTAAGATGGTCAT [CT] AGCTAC ATCCTGG P87S CACTTTCTTTGAGAGATCCCTT CTTTCCTCACAAGGGATCTCT AKR1B10 [CT] GTGAGGAAAG CAAAGAAAGTG M286T GTGATGAGGAGACGGCAACCA GAGTATGGTTGCCGTCTCCTC [TC] TACTC ATCAC N313D GACTATCCCTTCGATGCAGAA CAATATTCTGCATCGAAGGGA [AG] TATTG TAGTC R170H CCAACTTCAACCACAGGCAGC CTCCAGCTGCCTGTGGTTGAA AKR1C1 [GA] TGGAG GTTGG Q172L CTTCAACCGCAGGCTGCTGGA GGATCATCTCCAGCAGCCTGC [AT] GATGATCC GGTTGAAG F46Y CAATAGAAGCCGGGTACCACC GAATCAATATGGTGGTACCCG AKR1C2 [TA] ATATTTGATTC GCTTCTATTG JPET#173179 -

Computational Inferences of Mutations Driving Mesenchymal Differentiation in Glioblastoma
Computational Inferences of Mutations Driving Mesenchymal Differentiation in Glioblastoma James Chen Submitted in partial fulfillment of the requirements for the Doctor of Philosophy Degree in the Graduate School of Arts and Sciences Columbia University 2013 ! 2013 James Chen All rights reserved ABSTRACT Computational Inferences of Mutations Driving Mesenchymal Differentiation in Glioblastoma James Chen This dissertation reviews the development and implementation of integrative, systems biology methods designed to parse driver mutations from high- throughput array data derived from human patients. The analysis of vast amounts of genomic and genetic data in the context of complex human genetic diseases such as Glioblastoma is a daunting task. Mutations exist by the hundreds, if not thousands, and only an unknown handful will contribute to the disease in a significant way. The goal of this project was to develop novel computational methods to identify candidate mutations from these data that drive the molecular differentiation of glioblastoma into the mesenchymal subtype, the most aggressive, poorest-prognosis tumors associated with glioblastoma. TABLE OF CONTENTS CHAPTER 1… Introduction and Background 1 Glioblastoma and the Mesenchymal Subtype 3 Systems Biology and Master Regulators 9 Thesis Project: Genetics and Genomics 20 CHAPTER 2… TCGA Data Processing 23 CHAPTER 3… DIGGIn Part 1 – Selecting f-CNVs 33 Mutual Information 40 Application and Analysis 45 CHAPTER 4… DIGGIn Part 2 – Selecting drivers 52 CHAPTER 5… KLHL9 Manuscript 63 Methods 90 CHAPTER 5a… Revisions work-in-progress 105 CHAPTER 6… Discussion 109 APPENDICES… 132 APPEND01 – TCGA classifications 133 APPEND02 – GBM f-CNV list 136 APPEND03 – MES f-CNV candidate drivers 152 APPEND04 – Scripts 149 APPEND05 – Manuscript Figures and Legends 175 APPEND06 – Manuscript Supplemental Materials 185 i ACKNOWLEDGEMENTS I would like to thank the Califano Lab and my mentor, Andrea Califano, for their intellectual and motivational support during my stay in their lab. -

Wild-Type and Mutated IDH1/2 Enzymes and Therapy Responses
Oncogene (2018) 37:1949–1960 https://doi.org/10.1038/s41388-017-0077-z REVIEW ARTICLE Corrected: Correction Wild-type and mutated IDH1/2 enzymes and therapy responses 1,2,3 3 2 1 Remco J. Molenaar ● Jaroslaw P. Maciejewski ● Johanna W. Wilmink ● Cornelis J.F. van Noorden Received: 22 September 2017 / Revised: 2 November 2017 / Accepted: 7 November 2017 / Published online: 25 January 2018 © The Author(s) 2018. This article is published with open access Abstract Isocitrate dehydrogenase 1 and 2 (IDH1/2) are key enzymes in cellular metabolism, epigenetic regulation, redox states, and DNA repair. IDH1/2 mutations are causal in the development and/or progression of various types of cancer due to supraphysiological production of D-2-hydroxyglutarate. In various tumor types, IDH1/2-mutated cancers predict for improved responses to treatment with irradiation or chemotherapy. The present review discusses the molecular basis of the sensitivity of IDH1/2-mutated cancers with respect to the function of mutated IDH1/2 in cellular processes and their interactions with novel IDH1/2-mutant inhibitors. Finally, lessons learned from IDH1/2 mutations for future clinical applications in IDH1/2 wild-type cancers are discussed. 1234567890();,: Introduction production of D-2HG is essentially a gain of function that is exclusive to mutant IDH1/2 enzymes, it was quickly rea- Isocitrate dehydrogenase 1 and 2 (IDH1/2) are key enzymes lized that these frequently-occurring genetic alterations that function at a crossroads of cellular metabolism, epi- were promising targets for personalized anti-cancer therapy genetic regulation, redox states, and DNA repair. Mutations with small-molecule inhibitors [13]. -

(IDH1) Helps Regulate Catalysis and Ph Sensitivity
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.19.049387; this version posted April 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Mechanisms of pH-dependent IDH1 catalysis An acidic residue buried in the dimer interface of isocitrate dehydrogenase 1 (IDH1) helps regulate catalysis and pH sensitivity Lucas A. Luna1, Zachary Lesecq1, Katharine A. White2, An Hoang1, David A. Scott3, Olga Zagnitko3, Andrey A. Bobkov3, Diane L. Barber4, Jamie M. Schiffer5, Daniel G. Isom6, and Christal D. Sohl1,‡,§ From the 1Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, 92182; 2Harper Cancer Research Institute, Department of Chemistry and Biochemistry, University of Notre Dame, South Bend, IN, 46617; 3Sanford Burnham Prebys Medical Discovery Institute, La Jolla, CA, 92037; 4Department of Cell and Tissue Biology, University of California, San Francisco, CA, 94143; 5Janssen Research and Development, 3210 Merryfield Row, San Diego, CA, 92121; 6Department of Pharmacology, Sylvester Comprehensive Cancer Center, and Center for Computational Sciences, University of Miami, Miami, FL, 33136 Running title: Mechanisms of pH-dependent IDH1 catalysis ‡To whom correspondence should be addressed: Christal D. Sohl, Department of Chemistry and Biochemistry, San Diego State University, CSL 328, 5500 Campanile Dr., San Diego, California 92182, Email: [email protected]; Telephone: (619) 594-2053. §This paper is dedicated to the memory of our dear colleague and friend, Michelle Evon Scott (1990-2020). Keywords: enzyme kinetics, cancer, tumor metabolism, pH regulation, post-translational modification (PTM), buried ionizable residues ABSTRACT protonation states leading to conformational Isocitrate dehydrogenase 1 (IDH1) changes that regulate catalysis. -

Integrated Analysis of Cancer Stem Cells-Associated Lncrna-Mirna-Mrna Network for Ovarian Cancer Via Microarray and Gene Expression Omnibus Database
Integrated analysis of cancer stem cells-associated lncRNA-miRNA-mRNA network for ovarian cancer via microarray and Gene Expression Omnibus database Zheng Li Shengjing Hospital of China Medical University Zhijiao Wang Shengjing Hospital of China Medical University Yingying Zhou ( [email protected] ) Shengjing Hospital of China Medical University https://orcid.org/0000-0002-8098-1632 Research Keywords: Ovarian cancer stem cells (OCSCs), Competing endogenous RNAs (ceRNAs), MiR-146a-5p, LINC006654, NRP2. Posted Date: November 13th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-106454/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/39 Abstract Background: Cancer stem cells (CSCs) are associated with the recurrence, metastasis and chemoresistance of epithelial ovarian cancer. Competing endogenous RNAs (CeRNAs) play an important role in maintenance of ovarian cancer stem cell-like cells (OCSCs) characteristics. To construct a ceRNA regulatory network for OCSCs, microarray technology and Gene Expression Omnibus (GEO) database had been used. Human serous epithelial ovarian carcinoma cell line COC1 cells were treated with cisplatin and paclitaxel then maintained in stem cell conditions for 6 days to obtain CD117+/CD133+ cells (OCSCs). We identied the differentially expressed miRNAs (DEMs), lncRNA (DELs) and mRNA (DEGs) between OCSCs and COC1 by microarray and combined them with representative microarray proles in GEO Database. Results: According to the combination, 28 DEMs were identied at rst, and 452 DEGs were obtained combining with the predicted targets of these miRNAs and our mRNA microarray results. Up-regulated DEGs of them were signicantly enriched in ‘p53 signaling pathway’, ‘FoxO signaling pathway’ and ‘MicroRNAs in cancer’, whereas down-regulated DEGs were signicantly enriched in ‘Adherens junction’ and ‘Hepatitis C’ pathway. -

AKR1C1 Controls Cisplatin-Resistance in Head and Neck Squamous Cell
Chang et al. Journal of Experimental & Clinical Cancer Research (2019) 38:245 https://doi.org/10.1186/s13046-019-1256-2 RESEARCH Open Access AKR1C1 controls cisplatin-resistance in head and neck squamous cell carcinoma through cross-talk with the STAT1/3 signaling pathway Wei-Min Chang1, Yu-Chan Chang1, Yi-Chieh Yang1, Sze-Kwan Lin2, Peter Mu-Hsin Chang3,4*† and Michael Hsiao1,5*† Abstract Background: Cisplatin is the first-line chemotherapy used against most upper aerodigestive tract carcinomas. In head and neck cancer, sensitivity to cisplatin remains the key issue in treatment response and outcome. Genetic heterogeneity and aberrant gene expression may be the intrinsic factors that cause primary cisplatin-resistance. Methods: Combination of the HNSCC gene expression data and the cisplatin sensitivity results from public database. We found that aldo-keto reductase family 1 member C1 (AKR1C1) may be associated with cisplatin sensitivity in HNSCC treatment of naïve cells. We examined the AKR1C1 expression and its correlation with cisplatin IC50 and prognosis in patients. The in vitro and in vivo AKR1C1 functions in cisplatin-resistance through overexpression or knockdown assays, respectively. cDNA microarrays were used to identify the upstream regulators that modulate AKR1C1-induced signaling in HNSCC. Finally, we used the cigarette metabolites to promote AKR1C1 expression and ruxolitinib to overcome AKR1C1-induced cisplatin-resistance. Results: AKR1C1 positively correlates to cisplatin-resistance in HNSCC cells. AKR1C1 is a poor prognostic factor for recurrence and death of HNSCC patients. Silencing of AKR1C1 not only reduced in vitro IC50 but also increased in vivo cisplatin responses and vise versa in overexpression cells.