Part II Summary of Product Characteristics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medication Instructions for Allergy Patients
MEDICATION INSTRUCTIONS FOR ALLERGY PATIENTS Drugs which contain antihistamine or have antihistaminic effects can result in negative reactions to skin testing. As a result, it may not be possible to properly interpret skin test results, and testing may have to be repeated at a later date. While this list is extensive, it is NOT all inclusive (particularly of the various brand names). Discontinue ALL antihistamines including the following medications seven (7) days prior to skin testing (unless longer time specified): Antihistamines – Generic name (Brand name(s)): Cetirizine (Zyrtec, Zyrtec-D) Hydroxyzine (Vistaril, Atarax) Desloratadine (Clarinex) Levocetirizine (Xyzal) Fexofenadine (Allegra, Allegra-D) Loratadine (Claritin, Claritin-D, Alavert) Diphenhydramine (Aleve PM, Benadryl, Bayer P.M., Benylin, Contac P.M., Doans P.M, Excedrin PM, Legatrin P.M.. Nytol, Tylenol Nighttime, Unisom, Zzzquil) Chlorpheniramine (Aller-Chlor, Allerest, Alka Seltzer Plus, Chlor-Trimeton, Comtrex, Contac, Co-Pyronil, Coricidin, CTM, Deconamine, Dristan, Dura-tap, Naldecon, Ornade Spansules, Rondec, Sinutab, Teldrin, Triaminic, Triaminicin, Tylenol Allergy) Azatadine (Optimine, Trinalin) Doxylamine (Nyquil) Brompheniramine (Bromfed, Dimetane, Dimetapp) Meclizine (Antivert) Carbinoxamine (Clistin, Rondec) Pheniramine Clemastine (Tavist) Phenyltoloxamine (Nadecon) Cyclizine (Marezine) Promethazine (Phenergan) Cyprohepatidine (Periactin) (9 days) Pyrilamine (Mepyramine) Dexbrompheniramine (Drixoral) Quinacrine (Atabrine) Dexchlorpheniramine (Extendryl, Polaramine) -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

Histamine and Antihistamines Sites of Action Conditions Which Cause Release Aron H
Learning Objectives I Histamine Pharmacological effects Histamine and Antihistamines Sites of action Conditions which cause release Aron H. Lichtman, Ph.D. Diagnostic uses Associate Professor II Antihistamines acting at the H1 and H2 receptor Pharmacology and Toxicology Pharmacological effects Mechanisms of action Therapeutic uses Side effects and drug interactions Be familiar with the existence of the H3 receptor III Be able to describe the main mechanism of action of cromolyn sodium and its clinical uses Histamine Pharmacology First autacoid to be discovered. (Greek: autos=self; Histamine Formation akos=cure) Synthesized in 1907 Synthesized in mammalian tissues by Demonstrated to be a natural constituent of decarboxylation of the amino acid l-histidine mammalian tissues (1927) Involved in inflammatory and anaphylactic reactions. Local application causes swelling redness, and edema, mimicking a mild inflammatory reaction. Large systemic doses leads to profound vascular changes similar to those seen after shock or anaphylactic origin Histamine Stored in complex with: Heparin Chondroitin Sulfate Eosinophilic Chemotactic Factor Neutrophilic Chemotactic Factor Proteases 1 Conditions That Release Histamine 1. Tissue injury: Any physical or chemical agent that injures tissue, skin or mucosa are particularly sensitive to injury and will cause the immediate release of histamine from mast cells. 2. Allergic reactions: exposure of an antigen to a previously sensitized (exposed) subject can immediately trigger allergic reactions. If sensitized by IgE antibodies attached to their surface membranes will degranulate when exposed to the appropriate antigen and release histamine, ATP and other mediators. 3. Drugs and other foreign compounds: morphine, dextran, antimalarial drugs, dyes, antibiotic bases, alkaloids, amides, quaternary ammonium compounds, enzymes (phospholipase C). -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -
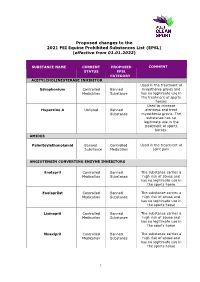
Proposed Changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (Effective from 01.01.2022)
Proposed changes to the 2021 FEI Equine Prohibited Substances List (EPSL) (effective from 01.01.2022) SUBSTANCE NAME CURRENT PROPOSED COMMENT STATUS EPSL CATEGORY ACETYLCHOLINESTERASE INHIBITOR Used in the treatment of Edrophonium Controlled Banned myasthenia gravis and Medication Substance has no legitimate use in the treatment of sports horses Used to increase Huperzine A Unlisted Banned alertness and treat Substance myasthenia gravis. The substance has no legitimate use in the treatment of sports horses. AMIDES Palmitoylethanolamid Banned Controlled Used in the treatment of Substance Medication joint pain ANGIOTENSIN CONVERTING ENZYME INHIBITORS Enalapril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Enalaprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Lisinopril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse Moexipril Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse 1 Perindoprilat Controlled Banned The substance carries a Medication Substance high risk of abuse and has no legitimate use in the sports horse ANTIHISTAMINES Antazoline Controlled Banned The substance has no Medication Substance legitimate use in the sports horse Azatadine Controlled Banned The substance has Medication Substance sedative effects -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Drug Schedules Regulation B.C
Pharmacy Operations and Drug Scheduling Act DRUG SCHEDULES REGULATION B.C. Reg. 9/98 Deposited and effective January 9, 1998 Last amended June 17, 2019 by B.C. Reg. 135/2019 Consolidated Regulations of British Columbia This is an unofficial consolidation. Point in time from June 17, 2019 to January 5, 2020 B.C. Reg. 9/98 (O.C. 35/98), deposited and effective January 9, 1998, is made under the Pharmacy Operations and Drug Scheduling Act, S.B.C. 2003, c. 77, s. 22. This is an unofficial consolidation provided for convenience only. This is not a copy prepared for the purposes of the Evidence Act. This consolidation includes any amendments deposited and in force as of the currency date at the bottom of each page. See the end of this regulation for any amendments deposited but not in force as of the currency date. Any amendments deposited after the currency date are listed in the B.C. Regulations Bulletins. All amendments to this regulation are listed in the Index of B.C. Regulations. Regulations Bulletins and the Index are available online at www.bclaws.ca. See the User Guide for more information about the Consolidated Regulations of British Columbia. The User Guide and the Consolidated Regulations of British Columbia are available online at www.bclaws.ca. Prepared by: Office of Legislative Counsel Ministry of Attorney General Victoria, B.C. Point in time from June 17, 2019 to January 5, 2020 Pharmacy Operations and Drug Scheduling Act DRUG SCHEDULES REGULATION B.C. Reg. 9/98 Contents 1 Alphabetical order 2 Sale of drugs 3 [Repealed] SCHEDULES Alphabetical order 1 (1) The drug schedules are printed in an alphabetical format to simplify the process of locating each individual drug entry and determining its status in British Columbia. -

2 12/ 35 74Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 22 March 2012 (22.03.2012) 2 12/ 35 74 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 9/16 (2006.01) A61K 9/51 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 9/14 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP201 1/065959 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 14 September 201 1 (14.09.201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/382,653 14 September 2010 (14.09.2010) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, NANOLOGICA AB [SE/SE]; P.O Box 8182, S-104 20 ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, Stockholm (SE). -

Use of Phenotypically Poor Metabolizer Individual Donor
DMD Fast Forward. Published on December 15, 2019 as DOI: 10.1124/dmd.119.089482 This article has not been copyedited and formatted. The final version may differ from this version. DMD #89486 Use of Phenotypically Poor Metaboliser Individual Donor Human Liver Microsomes to Identify Selective Substrates of UGT2B10 Nicolo Milani, Nahong Qiu, Birgit Molitor, Justine Badée, Gabriele Cruciani and Stephen Fowler Pharmaceutical Sciences, Roche Pharma Research and Early Development, Roche Innovation Centre Downloaded from Basel, Grenzacherstrasse 124, 4070, Basel, Switzerland (NM, NQ, BM, SF); Department of Chemistry, Biology and Biotechnology, University of Perugia, 06123 Perugia, Italy (NM, GC), Department of Pharmaceutics, Center for Pharmacometrics and Systems Pharmacology, University dmd.aspetjournals.org of Florida at Lake Nona, Orlando, Florida, USA (JB)* *Current Address: Modelling and Simulation, PK Sciences, Novartis Institutes for BioMedical Research, CH-4002, Basel, Switzerland. at ASPET Journals on September 23, 2021 1 DMD Fast Forward. Published on December 15, 2019 as DOI: 10.1124/dmd.119.089482 This article has not been copyedited and formatted. The final version may differ from this version. DMD #89486 Running title: Rapid detection of selective UGT2B10 substrates Corresponding author: Stephen Fowler, Pharmaceutical Sciences, Roche Pharma Research and Early Development, Roche Innovation Centre Basel, Grenzacherstrasse 124, 4070, Basel, Switzerland. Phone: +41 61 688 5105. Email:[email protected] Downloaded from Abbreviations: HLM: human liver microsomes, Km: Michaelis-Menten constant, MgCl2: magnesium chloride, LC: dmd.aspetjournals.org liquid chromatography, MS/MS: tandem mass spectrometry, UDPGA: uridine 5’- diphosphoglucuronic acid, UGT: Uridine 5’-diphospho (UDP)-glucuronosyltransferases, UHPLC: ultra-high performance liquid chromatography, NADPH: Nicotinamide adenine dinucleotide at ASPET Journals on September 23, 2021 phosphate, MIFs: Molecular Interaction Fields, Clint: intrinsic clearance, S.D.: standard deviation. -

The Relation Between Antihistamine Medication During Early Pregnancy & Birth Defects
The Egyptian Journal of Medical Human Genetics (2015) 16, 287–290 HOSTED BY Ain Shams University The Egyptian Journal of Medical Human Genetics www.ejmhg.eg.net www.sciencedirect.com REVIEW The relation between antihistamine medication during early pregnancy & birth defects Rabah M. Shawky a,*, Neveen S. Seifeldin b a Genetics Unit, Pediatric Department, Ain Shams University, Egypt b Dermatology, Venereology and Andrology Department, Ain Shams University, Egypt Received 6 April 2015; accepted 16 April 2015 Available online 11 May 2015 KEYWORDS Abstract Antihistamines are a group of medications which can inhibit various histaminic actions Antihistamine; at one of two histamine receptors (H1 or H2). H1 receptor antagonists are used for the relief of Birth defects; allergic dermatological and nondermatological conditions. We will review classes of antihistamines Congenital malformation; (H1 antagonists) and the relationship between specific antihistamines and specific birth defects. H1 antagonist; Although many findings provide reassurance about the relative safety of many antihistamine drugs Early pregnancy; and that any malformation reported is most probably caused by chance, studies are still required to Dermatological conditions assure fetal safety. As pruritus is sometimes troublesome for pregnant women topical medications like emollients should be tried first in the first trimester of pregnancy. Also pregnant women should be advised to consult their health care provider before taking any medication. Ó 2015 Production and hosting by Elsevier B.V. on behalf of Ain Shams University. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Contents 1. Introduction . 287 2. Classification of H1 antihistamines . -

Medicines That Adversely Affect Allergy Skin Testing
1 OSU Department of Otolaryngology–Head and Neck Surgery Division of Sinus and Allergy at the OSU Eye and Ear Institute 915 Olentangy River Rd. 4th Floor Columbus, Oh 43212 614-366-ENTS (3687) Medicines that affect allergy skin testing: Do not take these medications 5 days or longer (as listed below), prior to your appointment: *This is not an all-inclusive list, review all the ingredients on the package or ask your doctor or pharmacist.* Antihistamines - First Generation Generic Name Brand Names Azatadine Optimine Bromphenarimine BroveX, Dimetane, Lodrane Carbinoxamine Maleate Histex Pd, Palgic and Pediatex AHIST, Aller-Chlor, C.P.M, Chlo-Amine, Chlor-Allergy, Chlor-Mal, ChlorTrimeton, Chlorphen, Chlorpheniramine (6 days) Effidac-24, Histex, Ridraman Clemastine (10 days) Allerhist-1, Contact 12hr Allergy, Tavist-1 Cyproheptadine (11 days) Periactin Dexchlorpheniramine Polaramine Actifed Sinus Day, Aler-Tab, Allergy, AllergySinus, Allermax, Aler-Dryl, Altaryl, Banophren, Benadryl, Calm-Aid, Children's Allergy, Compoz Nighttime, Diphedryl, Diphen-Allergy, Diphenhist, Dormin Sleep Aide, Dytan, Dytuss, Genahist, Hydramine, Ibuprofen PM, Nu-Med, Nytol, PediaCare Diphenhydramine Children's Allergy, Q-Dryl, Quenalin, Scot-Tussin Allergy, Siladryl, Silphen, Simply Allergy, Simply Sleep, Sleep-ettes, Sleep Formula, Sleepinal, Sominex, Tavist, Theraflu, Triaminic, Twilite, Tylenol PM, Unisom Sleep Gels, Valu-Dryl Dimenhydrinate Dramamine Hydroxyzine (8 days) Atarax, Rezine, Vistaril Ketotifen Zatiden Meclizine HCl Antivert, Bonine Methdilazine -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data.