Chemical Characterization and Source Apportionment of PM2.5 in Rabigh, Saudi Arabia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Saudi Aramco Rabigh Refinery Control System
SUCCESS STORY Saudi Aramco Rabigh Refi nery Control System Replacement (Hot Cutover) Location: Rabigh, Kingdom of Saudi Arabia Order Date: October 2003 Completion Date: May 2006 Industry: Refi ning About Saudi Aramco and the Rabigh Refi nery Saudi Aramco’s operations span the globe and the energy industry. The world leader in crude oil production, Saudi Aramco also owns and operates an extensive network of refi ning and distribution facilities, and is responsible for gas processing and transportation installations that fuel Saudi Arabia’s industrial sector. An array of international subsidiaries and joint ventures deliver crude oil and refi ned products to customers worldwide. World-class refi neries located across the country, from the Arabian Gulf to the Red Sea, reliably supply more than a million barrels of products each day to meet the needs of the Saudi Arabian and international markets. The Rabigh Refi nery, located 160 kilometers north of Jeddah on the Rea Sea coast, is one such refi nery operated by Saudi Aramco. The Rabigh refi nery has a 400,000 BPD crude topping facility. Crude is delivered by tankers through the Saudi Aramco Rabigh port. The main products are fuel oil, naphtha, and jet fuel. LPG and oil are used as fuel for the refi nery while recovered sulphur is bagged and shipped. Background of This Project As part of an upgrade project to reap the benefi ts of the latest technology, Saudi Aramco Rabigh Refi nery awarded Yokogawa this project to replace the existing control system with a state-of-the-art distributed control system (DCS). -
Halaman 1 Dari 30 Muka | Daftar
Halaman 1 dari 30 muka | daftar isi Halaman 2 dari 30 muka | daftar isi Halaman 3 dari 30 Perpustakaan Nasional : Katalog Dalam terbitan (KDT) Miqat di Jeddah Tidak Sah? Penulis : Luki Nugroho, Lc 37 hlm ISBN 978-602-1989-1-9 Judul Buku Miqat di Jeddah Tidak Sah? Penulis Luki Nugroho, Lc. MA Editor Fatih Setting & Lay out Fayyad & Fawwaz Desain Cover Faqih Penerbit Rumah Fiqih Publishing Jalan Karet Pedurenan no. 53 Kuningan Setiabudi Jakarta Selatan 12940 Cet : Agustus 2018 muka | daftar isi Halaman 4 dari 30 Daftar Isi Daftar Isi ...................................................................................... 4 A. Permasalahan........................................................................... 7 a. Pangkal Masalah .............................................. 7 b. Perbedaan Pendapat Ulama ............................ 7 B. Pengertian Miqat ...................................................................... 8 1. Bahasa .............................................................. 8 2. Istilah ................................................................ 8 C. Miqat Makani ............................................................................ 9 1. Dzul Hulaifah .................................................. 12 2. Al-Juhfah ........................................................ 15 3. Qarnul Manazil ............................................... 15 4. Yalamlam ........................................................ 16 5. Dzatu ‘Irqin ..................................................... 17 D. Miqat Penumpang -

Investor Presentation
Investor update presentation September 2015 Content Introduction 4 Update on financial performance 6-11 Overview of E-Commerce initiatives 13-21 Update on Makkah investments 23-31 2 Section 1 Introduction Al Tayyar Travel Group Holding Co (ATG) at a glance • With market capitalization of about US$ 4.8 billion, ATG is the leading integrated travel service provider in the MENA region • ATG is the leading travel management service Top sales award Newly awarded Top agent award Silver award provider of corporate and government travel with (2009, 2010, 2011, Exclusive GSA (1999, 2002, 2004, (2010, 2011, interest in hospitality sector 2012, 2013) 2005, 2011, 2012 2012, 2013) • Enabled by a robust technology platform, ATG & 2013) serves its clients through a global network of more than 430 branches • ATG is building a strong position in the religious Passengers Sales Sale Excellence Top agent award Top sales agent fro tourism market in Makkah through a vertical award awards (2009, 2010, 2011, (2008, 2009, 2010, 2011, integration strategy owning large number of hotels (1994, 1995, 1996, (2009, 2010, 2011, 2012, 2013) 2012, 2013, 2014) in 2010, 2011, 2012 2012, 2013) Central province • ATG has consistently won prestigious awards and and 2013) recognitions from its partners and leading airlines Top low-cost carrier in the GCC ATG’s success story • IPO in June 2012 • Acquisition of • Acquisitions of • Acquisition of 100% of • Acquisition of Mawasim Elegant Resorts, Muthmerah Muthmerah Real CTM, and Al Hanove • Investment in Careem 2014 Estate company -

Late Pleistocene Raised Coral Reefs in the Eastern Red Sea – Rabigh, Saudi Arabia Ammar Manaa University of Wollongong
University of Wollongong Research Online University of Wollongong Thesis Collection University of Wollongong Thesis Collections 2011 Late Pleistocene raised coral reefs in the eastern red sea – Rabigh, Saudi Arabia Ammar Manaa University of Wollongong Recommended Citation Manaa, Ammar, Late Pleistocene raised coral reefs in the eastern red sea – Rabigh, Saudi Arabia, Master of Science - Research thesis, School of Earth and Environmental Sciences, University of Wollongong, 2011. http://ro.uow.edu.au/theses/3501 Research Online is the open access institutional repository for the University of Wollongong. For further information contact Manager Repository Services: [email protected]. Late Pleistocene raised coral reefs in the eastern Red Sea – Rabigh, Saudi Arabia *A thesis submitted in partial fulfilment of the requirements of the award of the degree MASTER OF SCIENCE (RESEARCH) From University of Wollongong By Ammar Manaa School of Earth and Environmental Sciences 2011 2 ABSTRACT The Rabigh coast (Saudi Arabia) in the study area stretches for about 12 km between Al Kharrar Lagoon in the north and Sharm Rabigh in the south. Seven prominent Pleistocene coral reef sites were investigated with terrace heights ranging from 1 to 5 m above present sea level. In addition to field descriptions, 86 samples were collected from these seven sites to provide the data for this research. Of these seven sites, 4 of the sites were front reef, and 3 were back reef. In each of the front reef sites, there was a beach rock, upper and lower reef. The elevation of the upper and lower reef in the front reef sites ranges from 0.5 m to 3.20 m above present sea level. -

Bab Iii Ikhtilaf Hukum Ibadat Haji Mengikut Pendapat Fuqaha Mazhab
BAB III IKHTILAF HUKUM IBADAT HAJI MENGIKUT PENDAPAT FUQAHA MAZHAB 3.1 PENGENALAN Roh ijtihad pada zaman imam mazhab yang menyala-nyala dengan hebatnya dianggap sebuah kelemahan. Ini kerana tumbuh suburnya pelbagai pendapat dan fahaman hukum. Bertitik tolak dari roh ijtihad itulah maka menghasilkan ajaran-ajaran hukum dan mazhab yang bercabang-cabang. Antara mazhab yang paling masyhur dan banyak jumlah pengikutnya terutama dalam kultur pemikiran hukum kaum sunni sehingga kini hanya empat iaitu, mazhab Hanafi, mazhab Maliki, mazhab Syafii, dan mazhab Hambali. Ijtihad adalah sebuah metode yang menemukan bentuk hukum terhadap kes tertentu yang secara harfiah tidak dijumpai dalam al-Quran dan sunnah. Mengenai metode penetapan hukum Islam pula terdiri daripada empat jenis: al-Quran, hadis, ijmak dan qiyas. Dalam hal ini, ijmak dan qiyas dijadikan sumber penetapan hukum dalam Islam kerana keduanya merupakan hasil pertimbangan fikiran dalam penetapan hukum berdasarkan al-Quran dan hadis. Keduanya juga memiliki cara yang sama dalam penetapan dasar hukum yang lainnya seperti istihsan, istislah dan istidlal. Kelima-lima cara penetapan hukum ini dalam perpustakaan hukum Islam dikenali sebagai al-ra‟y. Istilah ijtihad dengan ra‟y ditafsirkan oleh para ahli hukum sebagai metode mencari 87 hukum melalui fikiran yang dinamakan ijmak, qiyas, istishab, istislah, dan „urf. Hakikatnya, hukum Islam adalah bersumberkan naqliyyah dan „aqliyyah yang kemudiannya melahirkan sumber ketiga, yakni kashfiyyah, kebenaran yang bersumber dari intuisi (kebenaran intuitif). -

Petro Rabigh)
Rabigh Refining & Petrochemical Company (Petro Rabigh) 2020 Board of Directors’ Report Contents of 2020 Board of Directors’ Report Page 1. Implemented and non-implemented provisions of CMA’s Corporate 4 Governance Regulations, and justifications therefore 2. Names, qualifications, and experience of the Board and Committees Members 4 and Executive Management 3. Names of the companies inside and outside the Kingdom in which a Board 7 member is a member of their current or previous Board member or manager 4. Composition of the Board and classification of its members, as follows: 9 Executive Director, Non-Executive Director, or Independent Director 5. Procedure taken by the Board to inform its members, Non-Executive Directors in particular, of the shareholders' suggestions and remarks on the Company 9 and its performance 6. A brief description of the competencies and duties of the committees, such as the audit committee, the nomination committee and the remuneration committee indicating their names, names of their chairmen, names of their 9 members, the number of their respective meetings, dates of those meetings and the members' attendance details of each meeting 7. The means used by the Board to assess its performance, the performance of its committees and members and the external body which conducted the 12 assessment and its relation with the Company 8. Remuneration of the Board members and Executive Management 12 9. Punishment, penalty, precautionary procedure or preventive measure imposed on the Company by the Capital Market Authority or any other supervisory, regulatory or judiciary authority, describing the reasons for non-compliance, the 16 imposing authority and the measures undertaken to remedy and avoid such non-compliance in the future 10. -

International System Summary: SAUDI ARABIA
International System Summary: SAUDI ARABIA UIC Map of Saudi Arabia’s High-Speed Rail Lines Saudi Arabia is a large country located in the Middle East nation’s capital of Riyadh is the most populous city with between the Red Sea and Persian Gulf on the Arabian Pen- 4.725 million followed by Jeddah (3.2 million) and Mecca insula. In land area, Saudi Arabia ranks as the 13th largest (1.484 million). The country’s GDP of $676.7 billion ranks country in the world. Saudi Arabia borders the countries 24th in the world and the GDP per capita of $24,000 of Iraq, Jordan, Kuwait, Oman, Qatar, United Arab Emir- ranks 55th. ates, and Yemen. The country’s population of 26.5 million people ranks 46th in the world, with 82 percent of the Sources: The World Factbook population designated as residing in urban areas. The INTERNATIONAL HIGH-SPEED RAIL SYSTEM SUMMARY: SAUDI ARABIA | 1 SY STEM DESCRIPTION AND HISTORY The Saudi Railway Master Plan for the period of 2010 to 2040 calls for the strategic development of 19 individual rail lines with a total length of approximately 9,900 km (6,150 miles). These projects are classified into three stages of development, with the first stage covering the years 2010 to 2025; second stage covering the years 2026 to 2033; and the third stage covering the years 2034 to 2040. The first stage projects are considered high-priority and include the following projects according to the Saudi Railways Orga- nization: • The double line upgrade of the existing two conven- tional rail lines between Dammam and Riyadh. -

A Critical and Comparative Study of the Spoken Dialect of Badr and District in Saudi Arabia, M
A CRITICAL AND COMPARATIVE STUDY OF THE SPOKEN DIALECT OF THE NARB TRIBE IN SAUDI ARABIA A thesis presented to the University of Leeds Department of Semitic Studies by ALAYAN. MOHAMMED IL-HAZMY for The Degree of-Doctor of Philosophy April YFr fi xt ?031 This dissertation has never been submitted to this or any other University. PREFACE The aim of this thesis is to describe and study analytically the dialect of the Harb tribe, and to determine its position among the neighbouring tribes. Harb is a very large tribe occupying an extensive area of Saudi Arabia, and it was impracticable for one individual to survey every settlement. This would have occupied a lengthy period, and would best be done by a team of investigators, rather than an individual. Thus we have limited our investigation to-two"-selected'regions, which we believe to be representative, the first ranging from north-east Rabigh up to al-Madina (representing the speech of the Harb in the Hijaz), and the second ranging from al-Madina to al-Fawwara in al-Qasirn district (representing the speech of the Harb in Central Arabia). We have thus left out of consideration an area extending fromCOsfän to Räbigh, where some-. members-of the Harb, partic- ularly those of the Muabbad, Bishr and Zubaid clan live. We have been unable in the northern central region, to go as far as al-Quwära and Dukhnah. However, some Harbis from the unsurveyed area were met with in our regions, and samples of their speech were obtained and included. Within these limitations, however the datä'collected are substantial and it is hoped comprehensive enough to give a clear picture of the main features of the Harb dialect. -
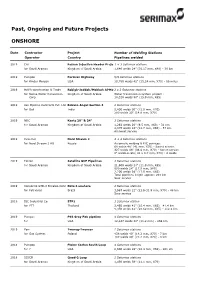
Past, Ongoing and Future Projects ONSHORE
Past, Ongoing and Future Projects ONSHORE Date Contractor Project Number of Welding Stations Operator Country Pipelines welded 2019 CAT Fazran Injection Header Proje 1 + 3 Saturnax stations for Saudi Aramco Kingdom of Saudi Arabia 1,640 welds 24" (30.17 mm, X60) - 39 km 2019 Pumpco Permian Highway 5/6 Saturnax stations for Kinder Morgan USA 10,780 welds 42" (15,24 mm, X70) - 66 miles 2019 MAPA construction & Trade Rabigh-Jeddah/Makkah Al-Mo 2 x 3 Saturnax stations for Saline Water Conversion Kingdom of Saudi Arabia Water transmission system project : Corp. 18,310 welds 80" (15.9 mm, X65) 2019 Ace Pipeline Contracts Pvt. Ltd Bokaro-Angul Section 3 2 Saturnax stations for Gail India 5,400 welds 30" (12.5 mm, X70) 200 welds 30" (14.8 mm, X70) 2019 NBC Kentz 20" & 24" 3 Saturnax stations for Saudi Aramco Kingdom of Saudi Arabia 1,292 welds 20" (9.5 mm, X60) - 31 km 2,375 welds 24" (12.7 mm, X60) - 57 km All sweet service 2019 Kvaerner Nord Stream 2 4 + 4 Saturnax stations for Nord Stream 2 AG Russia Automatic welding & FJC services: 68 welds 48" (41 mm, X70) - Sweet service 327 welds 48" (34.6 mm, X70) - Sweet service 2" welds-o-lets; 41 x 8.7 mm, X70) : 4 welds 2019 Tekfen Satellite GCP Pipelines 3 Saturnax stations for Saudi Aramco Kingdom of Saudi Arabia 21,600 welds 24" (11.9 mm, X65) 600 welds 24" (17.5 mm, X65) 2,200 welds 36" (17.5 mm, X65) Total pipelines length approx; 293 km Sour service 2019 Consorcio GTR-3 Encalso-Conc Rota 3 onshore 2 Saturnax stations for Petrobras Brazil 3,967 welds 22" (23.8-31.8 mm, X70) - 48 km Sour service 2019 IBC Industrial Co. -

Saudi Arabia's Economic Cities
Saudi Arabia’s Economic Cities Economic Cities Agency SAGIA Economic Cities: Pockets of Competitiveness Provide a comprehensive package to investors Mega initiative Economic City Concept New Concept Integrated cities 2 Creating Partnerships Global innovation in public private partnerships (PPP) Government Private Sector Regulator Capital provider Facilitator Land owner Promoter Developer 3 The Kingdom of Saudi Arabia has announced the launch of six economic cities Objective of the economic cities Eastern Province Tabouk To grow the national economy and Hail raise the standard of living for Saudis through: Medinah • Enhancing the competitiveness of the Saudi economy Rabigh • Creating new jobs • Improving Saudis’ skill levels • Developing the regions • Diversifying the Economy Jazan 4 Source: Team analysis To ensure success, the economic cities will be developed according to six key design principles Development based on globally State of the art ‘hard’ and ‘soft’ competitive advantage infrastructure • Each city will be developed around at least • The cities will utilize their greenfield one globally competitive cluster or industry, opportunity to adopt state-of-the-art which will serve as an anchor and a growth technology solutions to make them truly engine for the city, around which other competitive businesses will locate Creating opportunities for the Attracting core jobs private sector • Each city will be developed by the private • By Identifying and attracting core investors, sector, and will therefore generate major core -

R Kttti~I ~~1Il1 A.Tillj •
231 3 r Óti~~l~ (~6i~~~\1 ~ r kttti~i ~~1il1 A.tillJ • 1 1lllll!l!l ll li 1 T he Coopera ti ve Office For Call & G uidance At AI-Badiab UndN Thc Supcrvisic,n OfThc Minis1ry Oí liláJnk Affoirs Endowmco1 Guidancc: & Propag¡,tion P.().Box 24'l32 Ri>•adh 11456. rc-1 .f3308S8 - Fax 4301122 Ang Aklat na lto ay Lathalain at Kaloob ng: Islamic Society for Information & Propagation [ISIP] sa pakikipag-tulungan ng The Cooperative Office for Call Guidance in Badiah Ipinahihintulot ang paglimbag ng aklat na ito upang ipamahagi nang libre sa kundisyon na walang anumang gagawing pagbabago maliban sa pabalat nito. Ang sinuman na magnanais na ilimbag ito sa layuning ipagbili ay mangyaring makipag-ugnayan muna sa bumubuo sa lupon ng ISIP sa pagsasalin at pamamatnugot. ~I ~~lv-.(~.J,ll ~~I v-:ffu•V'-"'~ _;.)ü~dl.1.1 ~, ~ _;.)Ll-->=-:' cill.11 ~ /. _,J4,:i ~I ülll,, ~IJ .~1 ~L..._; l.l-"' J.:1.) J.....==::,1 . ..4,\ í "1''\ , _;,:,4.)1 - . ~ f-'""nxw, ú""\"1'• '\V A - 1 • 'I" -A· "1'\ - • A -A : ctl..A.)_; F' -, \ í"1''\/Wí\ °1'0°1',0 : '-fY..) \ H'\/1íí \ : t1...1;!)'1 ~_; '\VA -1•'1" -A•°1'\ -•A -A: ctl..A.)_; ------- - Masusing Gabay sa Pagsasagawa ng Umrah at Hajj ANG MGA NILALAMAN Paunang Salita 7 Úmrah at Hajj 9 And Kundisyon ng Umrah at Hajj 11 An~_Mga Kaasalan sa Úmrah at Hajj 13 Mga Bagay na Dapat lsaalang-alang Bago lsagawa 14 ang Úmrah at Hajj Mga Bagay na Dapat Isagawa Habang 17 N aglalakbar Mga Payo at Mungkahi Habang Nagsasagawa ng 19 Úmrah at Hajj AngUmrah 20 --- --. -

Saudi Arabia National Renewable Energy Program
Saudi Arabia National Renewable 1 Energy Program Saudi Arabia significantly increased its renewable energy targets and long term visibility Planned Capacity (GW) 5-Year Target 12-Year Target 58.7 Increased 2.7 CSP 5-Year Target 16.0 Wind Extended visibility 2 to 2030 27.3 7.0 Optimized 40.0 Solar PV the energy mix 9.5 20.0 2.4 Manufacturing 5.9 capacity of 200GW by Initial Revised 2030 Target 2030 12 pre-developed projects will be tendered in 2019 with a total capacity of ~3.1 GW Qurrayat Alfaisalia Saad Wadi Adwawser Yanbu TOTAL 200 600 600 70 850 Madinah Rabigh Alras Qurrayat 50 300 300 40 3 2,225 Rafha Jeddah 45 300 Mahad Dahab 850 20 Projects will be deployed in 35+ parks spread across the Kingdom Waad Al Shammal Qurayyat Tabarjal Rafha 35+ parks Sakaka North Tabuk Al Kahafah to be developed by 2030 Tabuk Midyan Qaisumah Al Masa'a Sourah Al Ghat Unaizah Sudair Spread across Al-Ula Ar Rass Shaqra Malham 4 Henakiyah Dhurma Ghilanah the Kingdom to promote Yanbu Khushaybi Tuwaiq Riyadh Al Haeer South Yanbu Madinah AlQuwaiiyah regional development Mastoorah Mahd Aldhab Duwadimi Rabigh Dhahban Starah Al-Kharj South Jeddah Al Faisaliah Haden Layla Al Laith Gradual deployment Bisha Wadi Ad Dawasir to mitigate technology risk Jazan Solar PV Farasan Sharorah Wind CSP What is Pre-Development? Site Selection Pre-development activities provide certainty and deliver lower project costs Energy Yield Grid Impact Assessment Studies Preliminary Pree-- Secure Design Input to tender DDeveellooppmmenetn Land 5 t AAccttivivitiiteises Reduced risk Successful IPP Hydrological Measure Assessment Resource Lower LCoE Geotech.