Cariprazine Exhibits Anxiolytic and Dopamine D Receptor-Dependent
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Efficacy of Antimanic Treatments: Meta-Analysis of Randomized, Controlled Trials
Neuropsychopharmacology (2011) 36, 375–389 & 2011 American College of Neuropsychopharmacology. All rights reserved 0893-133X/11 $32.00 www.neuropsychopharmacology.org Efficacy of Antimanic Treatments: Meta-analysis of Randomized, Controlled Trials ,1,2 2,3 4 2 Ays¸egu¨l Yildiz* , Eduard Vieta , Stefan Leucht and Ross J Baldessarini 1 2 Department of Psychiatry, Dokuz Eylu¨l University, Izmir, Turkey; Department of Psychiatry, Harvard Medical School and International Consortium for Bipolar Disorder Research and Psychopharmacology Program, McLean Division of Massachusetts General Hospital, Boston, 3 Massachusetts; Bipolar Disorders Program, Institute of Clinical Neuroscience, Hospital Clinic, University of Barcelona, IDIBAPS, CIBERSAM, 4 Barcelona, Spain; Department of Psychiatry and Psychotherapy, Klinik fu¨r Psychiatrie und Psychotherapie der TU-Mu¨nchen, Klinikum rechts der Isar, Technische Universita¨t Mu¨nchen, Mu¨nchen, Germany We conducted meta-analyses of findings from randomized, placebo-controlled, short-term trials for acute mania in manic or mixed states of DSM (III–IV) bipolar I disorder in 56 drug–placebo comparisons of 17 agents from 38 studies involving 10 800 patients. Of drugs tested, 13 (76%) were more effective than placebo: aripiprazole, asenapine, carbamazepine, cariprazine, haloperidol, lithium, olanzapine, paliperdone, quetiapine, risperidone, tamoxifen, valproate, and ziprasidone. Their pooled effect size for mania improvement (Hedges’ g in 48 trials) was 0.42 (confidence interval (CI): 0.36–0.48); pooled responder risk ratio (46 trials) was 1.52 (CI: 1.42–1.62); responder rate difference (RD) was 17% (drug: 48%, placebo: 31%), yielding an estimated number-needed-to-treat of 6 (all po0.0001). In several direct comparisons, responses to various antipsychotics were somewhat greater or more rapid than lithium, valproate, or carbamazepine; lithium did not differ from valproate, nor did second generation antipsychotics differ from haloperidol. -
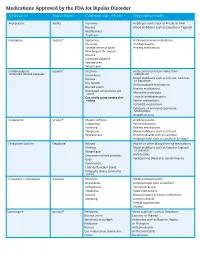
Medications Approved by the FDA for Bipolar Disorder Medication Brand Name Common Side Effects May Interact With
Medications Approved by the FDA for Bipolar Disorder Medication Brand Name Common side effects May interact with Aripiprazole Abilify® Insomnia Antidepressants such as Prozac or Paxil Nausea Mood stabilizers such as Equetro or Tegretol Restlessness Tiredness Asenapine Saphris® Sleepiness Antihypertensive medications Dizziness Antidepressants Strange sense of taste Anxiety medications Numbing of the mouth Nausea Increased appetite Feeling tired Weight gain Carbamazepine Equetro™ Dizziness Birth control pills (can make them extended release capsules Drowsiness ineffective) Mood stabilizers such as Lithium, Lamictal, Nausea or Depakote Dry mouth Anticonvulsant medications Blurred vision Anxiety medications Decreased white blood cell count Macrolide antibiotics Can rarely cause severe skin Tricyclic antidepressants rashes Cancer medications HIV/AIDS medications Cytotoxic or immunosuppressive Medications Grapefruit juice Cariprazine Vraylar® Muscle stiffness Antidepressants Indigestion Pain medications Vomiting Anxiety medications Sleepiness Mood stabilizers such as Lithium Restlessness Anticonvulsants such as Lamictal Antipsychotics such as Latuda or Seroquel Divalproex Sodium Depakote® Nausea Aspirin or other blood thinning medications Shaking Mood stabilizers such as Equetro,Tegretol, Weight gain or Lamictal Decrease in blood platelets Barbiturates Rash Cyclosporine (Neoral or Sandimmune) Pancreatitis Liver dysfunction (rare) Polycystic Ovary Syndrome (rare) Fluoxetine + Olanzapine Symbyax® Dizziness MAOI antidepressants Drowsiness Antipsychotics -
Psychotropic Drug Indications
PSYCHOTROPIC DRUG INDICATIONS (Part 1 of 2) Bipolar Disorder Mixed Generic Brand Form Mania Depression Episodes Maintenance MDD TRD PMDD Schizophrenia Others4 ATYPICAL ANTIPSYCHOTICS aripiprazole — tabs, ODT, oral √ √ √ √1 √ √ soln Abilify tabs √ √ √ √1 √ √ Abilify ext-rel IM inj √ √ Maintena Abilify tabs with √ √ √ √1 √ Mycite sensor aripiprazole Aristada ext-rel IM inj √ lauroxil Aristada ext-rel IM inj √ Initio asenapine Saphris sublingual tabs √ √ √ √ brexpiprazole Rexulti tabs √1 √ cariprazine Vraylar caps √ √ √ √ lurasidone Latuda tabs √ √ olanzapine Zyprexa tabs √ √2 √ √ √2 √ IM inj √ Zyprexa ext-rel IM inj √ Relprevv Zyprexa ODT √ √2 √ √ √2 √ Zydis quetiapine Seroquel tabs √ √ √ √ Seroquel XR ext-rel tabs √ √ √ √ √1 √ risperidone Perseris ext-rel SC inj √ Risperdal tabs, oral soln √ √ √ √ Risperdal ext-rel IM inj √ √ Consta Risperdal ODT √ √ √ √ M-tabs ziprasidone Geodon caps √ √ √1 √ IM inj √ COMBINATION ATYPICAL & SELECTIVE SEROTONIN REUPTAKE INHIBITOR olanzapine + Symbyax caps √ √ fluoxetine MONOAMINE OXIDASE INHIBITORS (MAOIs) phenelzine Nardil tabs √ selegiline EMSAM transdermal √ system tranylcypromine Parnate tabs √ SEROTONIN AND NOREPINEPHRINE REUPTAKE INHIBITORS (SNRIs) desvenlafaxine Khedezla ext-rel tabs √ Pristiq ext-rel tabs √ duloxetine Cymbalta caps √ √ levomilnacipran Fetzima ext-rel caps √ venlafaxine — scored tabs √ Effexor XR ext-rel caps √ √ SELECTIVE SEROTONIN REUPTAKE INHIBITORS (SSRIs) citalopram Celexa scored tabs √ escitalopram Lexapro scored tabs, √ √ oral soln fluoxetine — tabs, oral soln √3 √ √3 √ Prozac -

Medications to Be Avoided Or Used with Caution in Parkinson's Disease
Medications To Be Avoided Or Used With Caution in Parkinson’s Disease This medication list is not intended to be complete and additional brand names may be found for each medication. Every patient is different and you may need to take one of these medications despite caution against it. Please discuss your particular situation with your physician and do not stop any medication that you are currently taking without first seeking advice from your physician. Most medications should be tapered off and not stopped suddenly. Although you may not be taking these medications at home, one of these medications may be introduced while hospitalized. If a hospitalization is planned, please have your neurologist contact your treating physician in the hospital to advise which medications should be avoided. Medications to be avoided or used with caution in combination with Selegiline HCL (Eldepryl®, Deprenyl®, Zelapar®), Rasagiline (Azilect®) and Safinamide (Xadago®) Medication Type Medication Name Brand Name Narcotics/Analgesics Meperidine Demerol® Tramadol Ultram® Methadone Dolophine® Propoxyphene Darvon® Antidepressants St. John’s Wort Several Brands Muscle Relaxants Cyclobenzaprine Flexeril® Cough Suppressants Dextromethorphan Robitussin® products, other brands — found as an ingredient in various cough and cold medications Decongestants/Stimulants Pseudoephedrine Sudafed® products, other Phenylephrine brands — found as an ingredient Ephedrine in various cold and allergy medications Other medications Linezolid (antibiotic) Zyvox® that inhibit Monoamine oxidase Phenelzine Nardil® Tranylcypromine Parnate® Isocarboxazid Marplan® Note: Additional medications are cautioned against in people taking Monoamine oxidase inhibitors (MAOI), including other opioids (beyond what is mentioned in the chart above), most classes of antidepressants and other stimulants (beyond what is mentioned in the chart above). -

Currently Prescribed Psychotropic Medications
CURRENTLY PRESCRIBED PSYCHOTROPIC MEDICATIONS Schizophrenia Depression Anxiety Disorders 1st generation antipsychotics: Tricyclics: Atarax (hydroxyzine) Haldol (haloperidol), *Anafranil (clomipramine) Ativan (lorazepam) Haldol Decanoate Asendin (amoxapine) BuSpar (buspirone) Loxitane (loxapine) Elavil (amitriptyline) *Inderal (propranolol) Mellaril (thioridazine) Norpramin (desipramine) Keppra (levetiracetam) Navane (thiothixene) Pamelor (nortriptyline) *Klonopin (clonazepam) Prolixin (fluphenazine), Prolixin Sinequan (doxepin) Librium (chlordiazepoxide) Decanoate Spravato (esketamine) Serax (oxazepam) Stelazine (trifluoperazine) Surmontil (trimipramine) Thorazine (chlorpromazine) *Tenormin (atenolol) Tofranil (imipramine) MEDICATIONS PSYCHOTROPIC PRESCRIBED CURRENTLY Trilafon (perphenazine) Tranxene (clorazepate) Vivactil (protriptyline) Valium (diazepam) 2nd generation antipsychotics: Zulresso (brexanolone) Vistaril (hydroxyzine) Abilify (aripiprazole) Aristada (aripiprazole) SSRIs: Xanax (alprazolam) Caplyta (lumateperone) Celexa (citalopram) *Antidepressants, especially SSRIs, are also used in the treatment of anxiety. Clozaril (clozapine) Lexapro (escitalopram) Fanapt (iloperidone) *Luvox (fluvoxamine) Geodon (ziprasidone) Paxil (paroxetine) Stimulants (used in the treatment of ADD/ADHD) Invega (paliperidone) Prozac (fluoxetine) Invega Sustenna Zoloft (sertraline) Adderall (amphetamine and Perseris (Risperidone injectable) dextroamphetamine) Latuda (lurasidone) MAOIs: Azstarys(dexmethylphenidate Rexulti (brexpiprazole) Emsam (selegiline) -

Mechanism of Action of Cariprazine
CNS Spectrums (2016), 21, 123–127. © Cambridge University Press 2016 doi:10.1017/S1092852916000043 BRAINSTORMS—Clinical Neuroscience Update Mechanism of action of cariprazine Stephen M. Stahl ISSUE: Cariprazine is a new therapeutic agent recently approved for the treatment of both schizophrenia and manic or mixed episodes associated with bipolar disorder, and is under investigation for the treatment of both bipolar depression and major depressive disorder. arises: What differentiates cariprazine from other Take-Home Points agents in its class? ’ Cariprazine is a dopamine multifunctional agent, but with pharmacologic properties that differentiate it from other agents in the atypical antipsychotic class. Cariprazine Has About the Same Intrinsic Activity as Brexpiprazole, but Less than Aripiprazole ’ Cariprazine is a dopamine and serotonin (5HT) receptor “ ” partial agonist with intrinsic activity at the D2 dopamine The dopamine agonist spectrum goes from silent receptor, similar to that of another new agent, antagonism on the far left of Figure 2, which is pure “ ” brexpiprazole. antagonism without any agonist activity, to full agonism on the far right of Figure 2, which is the ’ High affinity actions of cariprazine at D3 dopamine maximum amount of stimulation of the D2 receptor.1 receptors, as well as actions at 5HT1A, 5HT2A, and Almost all antipsychotics are silent antagonists and lie alpha 1B receptors, differentiate it pharmacologically to the far left on the spectrum. Dopamine itself is a full from other antipsychotics. agonist and lies to the far right on the spectrum. Note that toward the left-hand part of the spectrum ’ Clinical differentiation from other antipsychotics is only lie several interesting compounds that have been tested now evolving with a head-to-head comparison with as antipsychotics. -

A Review of This Dopamine D3-Preferring D3/D2 Receptor Partial Agonist Leslie Citrome 1
New Drug Review Cariprazine for the Treatment of Schizophrenia: A Review of this Dopamine D3-Preferring D3/D2 Receptor Partial Agonist Leslie Citrome 1 Abstract Cariprazine is an antipsychotic medication and received approval by the U.S. Food and Drug Administration for the treatment of schizophrenia in September 2015. Cariprazine is a dopamine D3 and D2 receptor partial agonist, with a preference for the D3 receptor. Cariprazine is also a partial agonist at the serotonin 5-HT1A receptor and acts as an antagonist at 5-HT2B and 5-HT2A receptors. The recommended dose range of cariprazine for the treatment of schizophrenia is 1.5–6 mg/d; the starting dose of 1.5 mg/d is potentially therapeutic. Cariprazine is administered once daily and is primarily metabolized in the liver through the CYP3A4 enzyme system and, to a lesser extent, by CYP2D6. There are two active metabolites of note, desmethyl-cariprazine and didesmethyl-cariprazine; the latter’s half-life is substantially longer than that for cariprazine and systemic exposure to didesmethyl-cariprazine is several times higher than that for cariprazine. Three positive, 6-week, Phase 2/3, randomized controlled trials in acute schizophrenia dem- onstrated superiority of cariprazine over placebo. Pooled responder rates were 31% for cariprazine 1.5–6 mg/d vs. 21% for placebo, resulting in a number needed to treat (NNT) of 10. In a 26–72 week, randomized withdrawal study, significantly fewer patients relapsed in the cariprazine group compared with placebo (24.8% vs. 47.5%), resulting in an NNT of 5. The most commonly encountered adverse events (incidence ≥5% and at least twice the rate of placebo) are extrapyramidal symptoms (number needed to harm [NNH] 15 for cariprazine 1.5–3 mg/d vs. -

Antipsychotic Medications Age and Step Therapy
Market Applicability Market DC GA KY MD NJ NY WA Applicable X X X NA X X X Antipsychotic Medications Age and Step Therapy Override(s) Approval Duration Prior Authorization 1 year Quantity Limit Atypical Antipsychotics Comment Quantity Limit Abilify Mycite (aripiprazole Non-Preferred May be subject to quantity with sensor) limit MSB Abilify Use MSB criteria Aripiprazole tablets Preferred Aripiprazole oral Non-Preferred disintegrating tablets Aripiprazole solution Non-Preferred clozapine tablets Preferred MSB Clozaril Use MSB criteria Caplyta (lumateperone) Non-Preferred capsules Fanapt (iloperidone) tablets Fanapt (iloperidone) Non-Preferred Titration Pack FazaClo (clozapine) oral Non-Preferred disintegrating tablets MSB Geodon capsules Use MSB Criteria Ziprasidone capsules Preferred MSB Invega tablets Use MSB criteria Paliperidone ER tablets Preferred Latuda (lurasidone) tablets Non-Preferred MSB Risperdal tablets, oral Use MSB criteria solution MSB Risperdal M-tabs oral Use MSB criteria disintegrating tablets Risperidone oral tablets Preferred Risperidone solution Preferred Risperdal oral disintegrating Non-Preferred tablets Saphris (asenapine) Non-Preferred sublingual tablets Secuado (asenapine) patch Non-Preferred CRX-ALL-0562-20 PAGE 1 of 8 06/09/2020 This policy does not apply to health plans or member categories that do not have pharmacy benefits, nor does it apply to Medicare. Note that market specific restrictions or transition-of-care benefit limitations may apply. Market Applicability Market DC GA KY MD NJ NY WA Applicable X -

Vraylar (Cariprazine), for Treatment of Depressive Episodes Associated with Bipolar I Disorder (Bipolar Depression) in Adults
Vraylar® (cariprazine) – New indication • On May 28, 2019, Allergan and Gedeon Richter announced the FDA approval of Vraylar (cariprazine), for treatment of depressive episodes associated with bipolar I disorder (bipolar depression) in adults. • Vraylar is also approved in adults for the treatment of schizophrenia and acute treatment of manic or mixed episodes associated with bipolar I disorder. • There are nearly 11 million adults in the U.S. living with bipolar disorder, a condition that causes periods of severe changes in mood, energy, and activity levels. — Bipolar depression refers to the depressive episodes of bipolar I disorder. People living with bipolar I disorder can have manic and depressive episodes, as well as mixed episodes that feature both manic and depressive symptoms at the same time. — Depressive symptoms are three times more prevalent than manic symptoms. • The approval of Vraylar’s new indication was based on one 8-week and two 6-week placebo- controlled studies in patients with depressive episodes associated with bipolar I disorder. In each study, the primary endpoint was change from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) total score at the end of week 6. — In the 8-week study (N = 571), Vraylar 1.5 mg daily was superior to placebo at the end of week 6 on the MADRS total score (difference -4.0; 95% CI: -6.3, -1.6). — In the first 6-week study (N = 474), Vraylar 1.5 mg and 3 mg daily were superior to placebo at the end of week 6 on the MADRS total score (Vraylar 1.5 mg: difference -2.5; 95% CI: - 4.6, -0.4; Vraylar 3 mg: difference -3.0; 95% CI: -5.1, -0.9). -

Atypical Injectable Antipsychotics for Schizophrenia Or Bipolar Disorder: a Review of Clinical Effectiveness And
CADTH RAPID RESPONSE REPORT: SUMMARY WITH CRITICAL APPRAISAL Atypical Injectable Antipsychotics for Schizophrenia or Bipolar Disorder: A Review of Clinical Effectiveness and Cost-Effectiveness Service Line: Rapid Response Service Version: 1.0 Publication Date: January 3, 2019 Report Length: 20 Pages Authors: Tasha Narain, Caitlyn Ford Cite As: Atypical Injectable Antipsychotics for Schizophrenia or Bipolar Disorder: A Review of Clinical Effectiveness and Cost-Effectiveness. Ottawa: CADTH; 2019 Jan. (CADTH rapid response report: summary with critical appraisal). ISSN: 1922-8147 (online) Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services. While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. -

Myelogram-Medications-To-Avoid.Pdf
Medication to be held 48 Banaflex /skeletal muscle relaxer Clozaril/antischizophenia HOURS BEFORE AND 24 HOURS Bancap/contains caffei Cogentin/antidyskinectic/ant AFTER MYELOGRAM Banzel/seizure adjunct iemetic/antipsychotic Updated December 2016 BC powder/ contains caffeine Compazine/phenothiazine Belviq/appetite suppressant Concerta/CNS stimulant Abilify / antipsychotic Bevespi contains formoterol Contrave/antidepressant Acetophenazine/ antipsychotic Benzphetamine/appetite Cyclizine/ antiemetic Adderall/amphetamine and suppressant antivertigo agent dextroamphetamine Bismuth subcitrate potassium/ Cyclobenzaprine/muscle Adipex-P/stimulant/ anorectic mineral relaxer Adsuva/antidepressant Bonine/meclicine Cylert/ADHD agent Adphen/ /antidepressant Bontril/appetite suppressant Cymbalta/antidepressant/tri appetite suppressant Bontril PDM/appetite suppressant cyclic Adipost/ appetite suppressants, Bortezomib/antineoplastics Sympathomimetic (Systemic Brintxelli / antidepressant Adrafinil/cognitive enhancer Bucladin-S softab/antiemetic Dapex/appetite suppressant Alatrofloxacin/ antibacterial antivertigo Dayquil/contains caffeine Aliskiren Bucet/contains caffeine Darvon 65/contain caffeine Hemifumarate/antihypertensive Buclizine/antiemetic/antivertigo Daytrana/CNS stimulant direct renal inhibitor / Budeption/antidepressant Desipramine/tricyclic/antide Alsuma/s Buphenamine/appetite suppressant pressant Amaphen/ barbituate Buproban/antidepressantr Desoxyn/amphetamine Amitriptyline/ tricyclic BupropionBuffets/caffeine Desvenlafaxine succinate/ antidepressant -

Therapeutic Class Overview Atypical Antipsychotics
Therapeutic Class Overview Atypical Antipsychotics INTRODUCTION x Antipsychotic medications have been used for over 50 years to treat schizophrenia and a variety of other psychiatric disorders (Miyamato et al 2005). x Antipsychotic medications exert their effect in part by blocking D2 receptors. It is the blockade of these receptors in the mesolimbic pathway that is believed to contribute to desired antipsychotic effects, especially improvement of positive symptoms associated with schizophrenia (Farah 2005). x Antipsychotics are divided into 2 distinct classes based on their affinity for D2 and other neuroreceptors: typical antipsychotics, also called first generation antipsychotics (FGAs), and atypical antipsychotics, also called second generation antipsychotics (SGAs) (Miyamato et al 2005). x Atypical antipsychotics do not have a uniform pharmacology or mechanism of action; these differences likely account for the different safety and tolerability profiles of these agents (Clinical Pharmacology 2017). Clozapine is an antagonist at all dopamine receptors (D1-5), with lower affinity for D1 and D2 receptors and high affinity for D4 receptors. Aripiprazole acts as a partial agonist at the D2 receptor, functioning as an agonist when synaptic dopamine levels are low and as an antagonist when they are high. Brexpiprazole and cariprazine are partial agonists at D-2 and 5-HT1A receptors and antagonists at 5-HT2A receptors. The remaining atypical antipsychotics share the similarity of D2 and 5-HT2A antagonism, but differ in activity at other