Atypical Injectable Antipsychotics for Schizophrenia Or Bipolar Disorder: a Review of Clinical Effectiveness And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Original Research Published, Reproduced, Transmitted, Modified, Posted, Sold, Licensed, Or Used for Commercial Purposes
This work may not be copied, distributed, displayed, Original Research published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the Efficacy and Effectiveness of Depot publisher’s Terms & Conditions. Versus Oral Antipsychotics in Schizophrenia: Synthesizing Results Across Different Research Designs Noam Y. Kirson, PhD; Peter J. Weiden, MD; Sander Yermakov, MS; Wayne Huang, MPP; Thomas Samuelson, BA; Steve J. Offord, PhD; Paul E. Greenberg, MS, MA; and Bruce J. O. Wong, MD ABSTRACT he cornerstone of long-term maintenance therapy Objective: Nonadherence is a major challenge in schizophrenia Tof schizophrenia patients is relapse prevention. treatment. While long-acting (depot) antipsychotic medications are Relapse prevention is necessary—albeit not sufficient— often recommended to address adherence problems, evidence on for eventual successful rehabilitation.1 In practice, the the comparative effectiveness of depot versus oral antipsychotics is effectiveness of maintenance antipsychotic treatment is inconsistent. We hypothesize that this inconsistency could be due to often undermined by poor adherence to therapy. Not systematic differences in study design. This review evaluates the effect only is nonadherence the single greatest modifiable of study design on the comparative effectiveness of antipsychotic risk factor for relapse,2,3 it is also often undetected, formulations. The optimal use of different antipsychotic formulations resulting in lost opportunities to employ psychosocial in a general clinical setting depends on better understanding of the underlying reasons for differences in effectiveness across research interventions for adherence, as well as uncertainty as designs. to the relative contribution of lack of efficacy versus Data Sources: A PubMed literature review targeted English-language adherence problems to poor outcomes. -
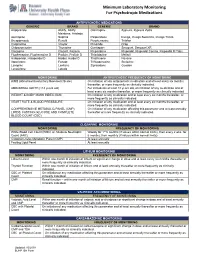
Minimum Laboratory Monitoring for Psychotropic Medications
Minimum Laboratory Monitoring For Psychotropic Medications ANTIPSYCHOTIC MEDICATIONS GENERIC BRAND GENERIC BRAND Aripiprazole Abilify, Abilify Olanzapine Zyprexa, Zyprexa Zydis Maintena, Aristada Asenapine Saphris Paliperidone Invega, Invega Sustenna, Invega Trinza Brexpiprazole Rexulti Perphenazine Trilafon Cariprazine Vraylar Pimozide Orap Chlorpromazine Thorazine Quetiapine Seroquel, Seroquel XR Clozapine Clozaril, Fazaclo Risperidone Risperdal, Risperdal Consta, Risperdal M Tabs Fluphenazine, Fluphenazine D Prolixin, Prolixin D Thioridazine Mellaril Haloperidol, Haloperidol D Haldol, Haldol D Thiothixene Navane Iloperidone Fanapt Trifluoperazine Stelazine Loxapine Loxitane Ziprasidone Geodon Lurasidone Latuda MONITORING ANTIPSYCHOTIC FREQUENCY OF MONITORING AIMS (Abnormal Involuntary Movement Scale) On initiation of any antipsychotic medication and at least every six months thereafter, or more frequently as clinically indicated. ABDOMINAL GIRTH (>18 years old) For individuals at least 18 years old, on initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. WEIGHT & BODY MASS INDEX (BMI) On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. HEART RATE & BLOOD PRESSSURE On initiation of any medication and at least every six months thereafter, or more frequently as clinically indicated. COMPREHENSIVE METABOLIC PANEL (CMP), On initiation of any medication affecting this parameter and at least annually LIPIDS, FASTING -

Drug Use Evaluation: Antipsychotic Utilization in Schizophrenia Patients
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Drug Use Evaluation: Antipsychotic Utilization in Schizophrenia Patients Research Questions: 1. How many schizophrenia patients are prescribed recommended first-line second-generation treatments for schizophrenia? 2. How many schizophrenia patients switch to an injectable antipsychotic after stabilization on an oral antipsychotic? 3. How many schizophrenia patients are prescribed 2 or more concomitant antipsychotics? 4. Are claims for long-acting injectable antipsychotics primarily billed as pharmacy or physician administered claims? 5. Does adherence to antipsychotic therapy differ between patients with claims for different routes of administration (oral vs. long-acting injectable)? Conclusions: In total, 4663 schizophrenia patients met inclusion criteria, and approximately 14% of patients (n=685) were identified as treatment naïve without claims for antipsychotics in the year before their first antipsychotic prescription. Approximately 45% of patients identified as treatment naïve had a history of remote antipsychotic use, but it is unclear if antipsychotics were historically prescribed for schizophrenia. Oral second-generation antipsychotics which are recommended as first-line treatment in the MHCAG schizophrenia algorithm were prescribed as initial treatment in 37% of treatment naive patients and 28% of all schizophrenia patients. Recommended agents include risperidone, paliperidone, and aripiprazole. Utilization of parenteral antipsychotics was limited in patients with schizophrenia. Overall only 8% of patients switched from an oral to an injectable therapy within 6 months of their first claim. Approximately, 60% of all schizophrenia patients (n=2512) had claims for a single antipsychotic for at least 12 continuous weeks and may be eligible to transition to a long-acting injectable antipsychotic. -

Schizophrenia Care Guide
August 2015 CCHCS/DHCS Care Guide: Schizophrenia SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT GOALS ALERTS Minimize frequency and severity of psychotic episodes Suicidal ideation or gestures Encourage medication adherence Abnormal movements Manage medication side effects Delusions Monitor as clinically appropriate Neuroleptic Malignant Syndrome Danger to self or others DIAGNOSTIC CRITERIA/EVALUATION (PER DSM V) 1. Rule out delirium or other medical illnesses mimicking schizophrenia (see page 5), medications or drugs of abuse causing psychosis (see page 6), other mental illness causes of psychosis, e.g., Bipolar Mania or Depression, Major Depression, PTSD, borderline personality disorder (see page 4). Ideas in patients (even odd ideas) that we disagree with can be learned and are therefore not necessarily signs of schizophrenia. Schizophrenia is a world-wide phenomenon that can occur in cultures with widely differing ideas. 2. Diagnosis is made based on the following: (Criteria A and B must be met) A. Two of the following symptoms/signs must be present over much of at least one month (unless treated), with a significant impact on social or occupational functioning, over at least a 6-month period of time: Delusions, Hallucinations, Disorganized Speech, Negative symptoms (social withdrawal, poverty of thought, etc.), severely disorganized or catatonic behavior. B. At least one of the symptoms/signs should be Delusions, Hallucinations, or Disorganized Speech. TREATMENT OPTIONS MEDICATIONS Informed consent for psychotropic -

Amisulpride Tablets I.P. SOLIAN® THERAPEUTIC CATEGORY Anti-Psychotic COMPOSITION Solian® 50 /100 /200 /400 Each Uncoated Tablet Contains Amisulpride IP
For the use only of a Registered Medical Practitioner (Psychiatrist) or a Hospital or a Laboratory Abridged Prescribing Information Amisulpride tablets I.P. SOLIAN® THERAPEUTIC CATEGORY Anti-psychotic COMPOSITION Solian® 50 /100 /200 /400 Each uncoated tablet contains Amisulpride IP. 50mg / 100mg / 200mg Each film coated tablet contains Amisulpride IP 400mg. THERAPEUTIC INDICATIONS Treatment of acute and chronic schizophrenic disorders, in which positive symptoms (such as delusions, hallucinations, and thought disorders) and/or negative symptoms (such as blunted affect, emotional and social withdrawal) are prominent, including patients characterised by predominant negative symptoms. DOSAGE AND ADMINISTRATION For acute psychotic episodes, oral doses between 400 and 800 mg/d are recommended. Doses above 1200 mg/d should not be used. For patients with mixed positive and negative symptoms, doses should be adjusted to obtain optimal control of positive symptoms. Maintenance treatment should be established individually with the minimally effective dose. For patients characterised by predominant negative symptoms, oral doses between 50 mg/d and 300 mg/d are recommended. Doses should be adjusted individually. Solian® can be administered once daily at oral doses up to 300 mg, higher doses should be administered bid. The Minimum effective dose should be used. Caution in elderly. Renal & Hepatic insufficiency: Dose should be reduced. Use of amisulpride from puberty to 18 years is not recommended. SAFETY-RELATED INFORMATION Contraindications: Hypersensitivity to amisulpride or to other ingredients of the product; concomitant prolactin- dependent tumours e.g. pituitary gland prolactinomas and breast cancer; phaeochromocytoma; children up to puberty; lactation; combinations with drugs which could induce torsades de pointes and levodopa. -

Aripiprazole Augmentation of Clomipramine Therapy In
Dusunen Adam The Journal of Psychiatry and Neurological Sciences 2016;29:167-172 Case Report / Olgu Sunumu DOI: 10.5350/DAJPN2016290209 Aripiprazole Augmentation Filiz Izci1, Murat Yalcin2, Sumeyye Yasemin Kurtulus Calli2, of Clomipramine Therapy in Yagmur Sever3, Rabia Bilici3 1Istanbul Bilim University, Faculty of Medicine, Treatment-Resistant Department of Psychiatry, Istanbul - Turkey 2Kocaeli Derince Training and Research Hospital, Department of Psychiatry, Kocaeli - Turkey Obsessive-Compulsive 3Erenkoy Training and Research Hospital for Psychiatric and Neurological Disorders, Istanbul - Turkey Disorder: Case Series ABSTRACT Aripiprazole augmentation of clomipramine therapy in treatment-resistant obsessive-compulsive disorder: case series Obsessive-compulsive disorder (OCD) is a chronic disorder characterized by recurrent intrusive thoughts and repetitive rituals, causing significant distress and functional loss. Studies show evidence about serotonergic and dopaminergic mechanisms in neuropathogenesis of OCD. Selective serotonin re-uptake inhibitors (SSRI) are considered as first-line treatment in OCDs, but treatment resistance may occur in 40-60% of cases treated with SSRIs. Augmentation of antidepressants with atypical antipsychotics is an important treatment option in treatment-resistant patients with OCD. In this article, we aimed to present five OCD cases with treatment-resistance in which we obtained good outcomes, with addition of aripiprazole 10-30mg per day to clomipramine therapy. Address reprint requests to / Yazışma adresi: -

Revision of Precautions Asenapine Maleate, Aripiprazole, Olanzapine
Published by Translated by Ministry of Health, Labour and Welfare Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. Revision of Precautions Asenapine maleate, aripiprazole, olanzapine, quetiapine fumarate, clocapramine hydrochloride hydrate, chlorpromazine hydrochloride, chlorpromazine hydrochloride/promethazine hydrochloride/phenobarbital, chlorpromazine phenolphthalinate, spiperone, zotepine, timiperone, haloperidol, paliperidone, pipamperone hydrochloride, fluphenazine decanoate, fluphenazine maleate, brexpiprazole, prochlorperazine maleate, prochlorperazine mesilate, Pharmaceuticals and Medical Devices Agency Office of Safety I 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan E-mail: [email protected] Published by Translated by Ministry of Health, Labour and Welfare Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. propericiazine, bromperidol, perphenazine, perphenazine hydrochloride, perphenazine fendizoate, perphenazine maleate, perospirone hydrochloride hydrate, mosapramine hydrochloride, risperidone (oral drug), levomepromazine hydrochloride, levomepromazine maleate March 27, 2018 Non-proprietary name Asenapine maleate, -

Current P SYCHIATRY
Current p SYCHIATRY N ew Investigators Tips to manage and prevent discontinuation syndromes Informed tapering can protect patients when you stop a medication Sriram Ramaswamy, MD Shruti Malik, MBBS, MHSA Vijay Dewan, MD Instructor, department of psychiatry Foreign medical graduate Assistant professor Creighton University Department of psychiatry Omaha, NE University of Nebraska Medical Center Omaha, NE bruptly stopping common psychotropics New insights on psychotropic A —particularly antidepressants, benzodi- drug safety and side effects azepines, or atypical antipsychotics—can trigger a discontinuation syndrome, with: This paper was among those entered in the 2005 • rebound or relapse of original symptoms Promising New Investigators competition sponsored • uncomfortable new physical and psycho- by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition logical symptoms was “New insights on psychotropic drug safety and • physiologic withdrawal at times. side effects.” To increase health professionals’ awareness of URRENT SYCHIATRY 1 C P is honored to publish this peer- the risk of these adverse effects, this article reviewed, evidence-based article on a clinically describes discontinuation syndromes associated important topic for practicing psychiatrists. with various psychotropics and offers strategies to NMSIS is dedicated to reducing morbidity and anticipate, recognize, and manage them. mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing -

Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment for Schizophrenia in Adult Patients? Kyle J
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Student Dissertations, Theses and Papers Scholarship 2017 Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles Philadelphia College of Osteopathic Medicine Follow this and additional works at: https://digitalcommons.pcom.edu/pa_systematic_reviews Part of the Psychiatry Commons Recommended Citation Knowles, Kyle J., "Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients?" (2017). PCOM Physician Assistant Studies Student Scholarship. 381. https://digitalcommons.pcom.edu/pa_systematic_reviews/381 This Selective Evidence-Based Medicine Review is brought to you for free and open access by the Student Dissertations, Theses and Papers at DigitalCommons@PCOM. It has been accepted for inclusion in PCOM Physician Assistant Studies Student Scholarship by an authorized administrator of DigitalCommons@PCOM. For more information, please contact [email protected]. Is Aristada (Aripiprazole Lauroxil) a Safe and Effective Treatment For Schizophrenia In Adult Patients? Kyle J. Knowles, PA-S A SELECTIVE EVIDENCE BASED MEDICINE REVIEW In Partial Fulfillment of the Requirements For The Degree of Master of Science In Health Sciences- Physician Assistant Department of Physician Assistant Studies Philadelphia College of Osteopathic Medicine Philadelphia, Pennsylvania December 16, 2016 ABSTRACT OBJECTIVE: The objective of this selective EBM review is to determine whether or not “Is Aristada (aripiprazole lauroxil) a safe and effective treatment for schizophrenia in adult patients?” STUDY DESIGN: Review of three randomized controlled studies. All three trials were conducted between 2014 and 2015. DATA SOURCES: One randomized, controlled trial and two randomized, controlled, double- blind trials found via Cochrane Library and PubMed. -

Antipsychotic Combinations
Graylands Hospital DRUG BULLETIN Pharmacy Department Brockway Road Mount Claremont WA 6010 Telephone (08) 9347 6400 Email [email protected] Fax (08) 9384 4586 Antipsychotic Combinations Graylands Hospital Drug Bulletin 2008 Vol. 15 No. 3 February ISSN 1323-1251 Antipsychotic combinations Reasons for antipsychotic combinations Despite the development of efficacious medications for the treatment of schizophrenia, many people do There are a number of theoretical benefits and not respond adequately. To address this problem, reasons cited for antipsychotic combination the use of two or more antipsychotics simultaneously prescribing, these include: is a commonly employed treatment strategy. Although the use of combination antipsychotics is Complementary mechanisms of action4 (e.g. common in clinical practice, the risks and benefits adding an antipsychotic with strong dopamine have not been systematically evaluated to date. As a D2 blockade to a weak D2 blocker) result, current Australian treatment algorithms Partial replacement of antipsychotic action for including the Royal Australian and New Zealand drugs with intolerable adverse effects at higher College of Psychiatry Schizophrenia Guidelines and 5 doses (e.g. adding quetiapine to clozapine to the Western Australian Therapeutic Advisory Group minimise metabolic adverse effects) Antipsychotic Guidelines advise against the use of combined antipsychotics, except for short periods of Alternative where clozapine cannot be used6 changeover1,2. Most data on antipsychotic -

Doxepin Exacerbates Renal Damage, Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Urinary Chromium Loss in Obese Mice
pharmaceuticals Article Doxepin Exacerbates Renal Damage, Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Urinary Chromium Loss in Obese Mice Geng-Ruei Chang 1,* , Po-Hsun Hou 2,3, Wei-Cheng Yang 4, Chao-Min Wang 1 , Pei-Shan Fan 1, Huei-Jyuan Liao 1 and To-Pang Chen 5,* 1 Department of Veterinary Medicine, National Chiayi University, 580 Xinmin Road, Chiayi 60054, Taiwan; [email protected] (C.-M.W.); [email protected] (P.-S.F.); [email protected] (H.-J.L.) 2 Department of Psychiatry, Taichung Veterans General Hospital, 1650 Taiwan Boulevard (Section 4), Taichung 40705, Taiwan; [email protected] 3 Faculty of Medicine, National Yang-Ming University, 155 Linong Street (Section 2), Taipei 11221, Taiwan 4 School of Veterinary Medicine, National Taiwan University, 1 Roosevelt Road (Section 4), Taipei 10617, Taiwan; [email protected] 5 Division of Endocrinology and Metabolism, Show Chwan Memorial Hospital, 542 Chung-Shan Road (Section 1), Changhua 50008, Taiwan * Correspondence: [email protected] (G.-R.C.); [email protected] (T.-P.C.); Tel.: +886-5-2732946 (G.-R.C.); +886-4-7256166 (T.-P.C.) Abstract: Doxepin is commonly prescribed for depression and anxiety treatment. Doxepin-related disruptions to metabolism and renal/hepatic adverse effects remain unclear; thus, the underlying mechanism of action warrants further research. Here, we investigated how doxepin affects lipid Citation: Chang, G.-R.; Hou, P.-H.; change, glucose homeostasis, chromium (Cr) distribution, renal impairment, liver damage, and fatty Yang, W.-C.; Wang, C.-M.; Fan, P.-S.; liver scores in C57BL6/J mice subjected to a high-fat diet and 5 mg/kg/day doxepin treatment for Liao, H.-J.; Chen, T.-P. -

SAFETY of the ELECTROCONVULSIVE THERAPY and AMISULPRIDE COMBINATION Rozália Takács1, Zsolt Iványi2, Gabor S
Psychiatria Danubina, 2013; Vol. 25, No. 1, pp 76-79 Brief report © Medicinska naklada - Zagreb, Croatia SAFETY OF THE ELECTROCONVULSIVE THERAPY AND AMISULPRIDE COMBINATION Rozália Takács1, Zsolt Iványi2, Gabor S. Ungvari3, 4 & Gábor Gazdag1,5 1Department of Psychiatry and Psychotherapy, Faculty of Medicine, Semmelweis University, Budapest, Hungary 2 Department of Anesthesiology and Intensive Therapy, Faculty of Medicine, Semmelweis University, Budapest, Hungary 3University of Notre Dame, Australia 4Marian Centre, Perth, Australia 5Consultation–Liaison Psychiatric Service, Szent István and Szent László Hospitals, Budapest, Hungary received: 21.10.2011; revised: 16.1.2012; accepted: 2.12.2012 SUMMARY Background: Electroconvulsive therapy is frequently considered when pharmacotherapy is ineffective. In such cases the combination of the two treatment modalities are commonly used. Amisulpiride, a second generation antipsychotic drug is used in the treatment of schizophrenia and psychotic depression. When amisulpiride is ineffective as a monotherapy, combination with ECT could be an option to enhance its efficacy. To the best of our knowledge, to date there have been no data about the safety of this combination. Subjects and methods: Medical notes of all patients who were given ECT while on amisulpiride were selected from the archives of the Department of Psychiatry, Semmelweis University Medical School, Budapest, covering a 10-year period. A randomly selected matched control group was formed from patients who underwent ECT but were not taking amisulpiride. Patients in both groups also received a variety of psychotropic drugs other than amisulpide. Side effects were compared between the two groups of patients. Results: Twenty patients received amisulpride with ECT. The most common side effects were headache, hypertension, tachycardia, nausea, dizziness, confusion, psychomotor agitation, sialorrhea, and prolonged seizure activity.