BMC Evolutionary Biology Biomed Central
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ED45E Rare and Scarce Species Hierarchy.Pdf
104 Species 55 Mollusc 8 Mollusc 334 Species 181 Mollusc 28 Mollusc 44 Species 23 Vascular Plant 14 Flowering Plant 45 Species 23 Vascular Plant 14 Flowering Plant 269 Species 149 Vascular Plant 84 Flowering Plant 13 Species 7 Mollusc 1 Mollusc 42 Species 21 Mollusc 2 Mollusc 43 Species 22 Mollusc 3 Mollusc 59 Species 30 Mollusc 4 Mollusc 59 Species 31 Mollusc 5 Mollusc 68 Species 36 Mollusc 6 Mollusc 81 Species 43 Mollusc 7 Mollusc 105 Species 56 Mollusc 9 Mollusc 117 Species 63 Mollusc 10 Mollusc 118 Species 64 Mollusc 11 Mollusc 119 Species 65 Mollusc 12 Mollusc 124 Species 68 Mollusc 13 Mollusc 125 Species 69 Mollusc 14 Mollusc 145 Species 81 Mollusc 15 Mollusc 150 Species 84 Mollusc 16 Mollusc 151 Species 85 Mollusc 17 Mollusc 152 Species 86 Mollusc 18 Mollusc 158 Species 90 Mollusc 19 Mollusc 184 Species 105 Mollusc 20 Mollusc 185 Species 106 Mollusc 21 Mollusc 186 Species 107 Mollusc 22 Mollusc 191 Species 110 Mollusc 23 Mollusc 245 Species 136 Mollusc 24 Mollusc 267 Species 148 Mollusc 25 Mollusc 270 Species 150 Mollusc 26 Mollusc 333 Species 180 Mollusc 27 Mollusc 347 Species 189 Mollusc 29 Mollusc 349 Species 191 Mollusc 30 Mollusc 365 Species 196 Mollusc 31 Mollusc 376 Species 203 Mollusc 32 Mollusc 377 Species 204 Mollusc 33 Mollusc 378 Species 205 Mollusc 34 Mollusc 379 Species 206 Mollusc 35 Mollusc 404 Species 221 Mollusc 36 Mollusc 414 Species 228 Mollusc 37 Mollusc 415 Species 229 Mollusc 38 Mollusc 416 Species 230 Mollusc 39 Mollusc 417 Species 231 Mollusc 40 Mollusc 418 Species 232 Mollusc 41 Mollusc 419 Species 233 -

Why Do Snails Have Hairs? a Bayesian Inference of Character Evolution Markus Pfenninger*1, Magda Hrabáková2, Dirk Steinke3 and Aline Dèpraz4
BMC Evolutionary Biology BioMed Central Research article Open Access Why do snails have hairs? A Bayesian inference of character evolution Markus Pfenninger*1, Magda Hrabáková2, Dirk Steinke3 and Aline Dèpraz4 Address: 1Abteilung Ökologie & Evolution, J.W. Goethe-Universität, BioCampus Siesmayerstraße, 60054 Frankfurt/Main, Germany, 2Deparment of Zoology, Charles University, Viniènà 7, 128 44 Praha 2, Czech Republic, 3Department of Biology, University of Konstanz, Postbox 5560 M618, 78457 Konstanz, Germany and 4Département d'Ecologie et Evolution, Université de Lausanne, Bâtiment de Biologie, Dorigny, 1015 Lausanne, Switzerland Email: Markus Pfenninger* - [email protected]; Magda Hrabáková - [email protected]; Dirk Steinke - [email protected]; Aline Dèpraz - [email protected] * Corresponding author Published: 04 November 2005 Received: 14 July 2005 Accepted: 04 November 2005 BMC Evolutionary Biology 2005, 5:59 doi:10.1186/1471-2148-5-59 This article is available from: http://www.biomedcentral.com/1471-2148/5/59 © 2005 Pfenninger et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Costly structures need to represent an adaptive advantage in order to be maintained over evolutionary times. Contrary to many other conspicuous shell ornamentations of gastropods, the haired shells of several Stylommatophoran land snails still lack a convincing adaptive explanation. In the present study, we analysed the correlation between the presence/absence of hairs and habitat conditions in the genus Trochulus in a Bayesian framework of character evolution. -

Plicuteria Lubomirski (Œlósarski, 1881)
Folia Malacol. 21(2): 91–97 http://dx.doi.org/10.12657/folmal.021.010 PLICUTERIA LUBOMIRSKI (ŒLÓSARSKI, 1881) (GASTROPODA: PULMONATA: HYGROMIIDAE), A FORGOTTEN ELEMENT OF THE ROMANIAN MOLLUSC FAUNA, WITH NOTES ON THE CORRECT SPELLING OF ITS NAME 1,* 2 3 4 BARNA PÁLL-GERGELY ,ROLAND FARKAS ,TAMÁS DELI ,FRANCISCO WELTER-SCHULTES 1 Department of Biology, Shinshu University, Matsumoto 390-8621, Japan (e-mail: [email protected]) 2 Aggtelek National Park Directorate, Tengerszem oldal 1, H-3758 Jósvafõ, Hungary (e-mail: [email protected]) 3 Békés Megyei Múzeumok Igazgatósága, Gyulai u 1., H-5600 Békéscsaba, Hungary (e-mail: [email protected]) 4 Zoologisches Institut, Berliner Strasse 28, 37073 Göttingen, Germany (e-mail: [email protected]) * corresponding author ABSTRACT: In this paper two new localities of the hygromiid land snail Plicuteria lubomirski (Œlósarski, 1881) are reported from Romania (Suceava and Harghita Counties). Its presence at the Lacu Roºu (Gyilkos-tó) area rep- resents the southeasternmost occurrence of the species. The only sample of P. lubomirski hitherto reported from Romania (leg. JICKELI in 1888 at Borsec Bai) seems to be lost from museum collections. The northern Carpathian distributional type is suggested for the species. The nomenclatural problem regarding the spelling of the specific name (i.e. lubomirskii or lubomirski) is discussed. The original, but less frequently used spelling (lubomirski) is suggested based on our understanding of the regulations of the ICZN. KEY WORDS: Carpathian species, faunistics, biogeography, Trochulus, anatomy INTRODUCTION Plicuteria lubomirski (Œlósarski, 1881) (often referred Umgebung des Bades Borszék” (=around Borsec Bai). to as Trochulus) is a Carpathian species reported from This record was later mentioned by SOÓS (1943) and Austria (KLEMM 1973, but also see REISCHÜTZ & GROSSU (1983). -

An Anomaly of the Reproductive Organs in Trochulus Hispidus (Linnaeus, 1758) (Gastropoda: Pulmonata: Hygromiidae)
Vol. 20(1): 39–41 doi: 10.2478/v10125-012-0002-6 AN ANOMALY OF THE REPRODUCTIVE ORGANS IN TROCHULUS HISPIDUS (LINNAEUS, 1758) (GASTROPODA: PULMONATA: HYGROMIIDAE) 1 2 MA£GORZATA PROÆKÓW ,EL¯BIETA KUZNIK-KOWALSKA 1Museum of Natural History, Wroc³aw University, Sienkiewicza 21, 50-335 Wroc³aw, Poland (e-mail: [email protected]) 2Department of Invertebrate Systematics and Ecology, Institute of Biology, Wroc³aw University of Environmental and Life Sciences, Ko¿uchowska 5b, 51-631 Wroc³aw, Poland ABSTRACT: A specimen of Trochulus hispidus from Buchenbach, Germany, had its male and female parts of the reproductive system completely separated. The female part lacked auxiliary organs. The male part opened into the genital atrium, but the vas deferens was blind-ended in a way precluding sperm transfer. KEY WORDS: land snail, Trochulus hispidus, Hygromiidae, reproductive organs, anomaly Anomalies of the reproductive organs have been did not differ from the remaining specimens from recorded in several species of pulmonate land snails. that site. On dissection, we found the reproductive or- The vast majority involves the male part of the repro- gans to be abnormal. Two separate parts could be dis- ductive system, while female organs are usually nor- tinguished. One was blind-ended and consisted of the mally developed. Repetition of the penis is the most gonad, the hermaphrodite duct, the albumen gland common anomaly and has been reported in Helix and the spermoviduct which passed into the short pomatia Linnaeus, 1758 (PARAVICINI 1898, PÉGOT spermathecal duct ending with the spermatheca (Fig. 1900, ASHWORTH 1907, LATTMANN 1967), Cernuella 1A). -

Malacologica
FOLIA Folia Malacol. 24(4): 265–273 MALACOLOGICA ISSN 1506-7629 The Association of Polish Malacologists Faculty of Biology, Adam Mickiewicz University Bogucki Wydawnictwo Naukowe Poznań, December 2016 http://dx.doi.org/10.12657/folmal.024.022 REPRODUCTIVE BIOLOGY AND GROWTH OF TWO VALLONIA SPECIES IN LABORATORY CONDITIONS (GASTROPODA: EUPULMONATA: VALLONIIDAE) ElżbiEta KuźniK-KowalsKa1*, Małgorzata ProćKów2 1Department of Invertebrate Systematics and Ecology, Institute of Biology, Wrocław University of Environmental and Life Sciences, Kożuchowska 5b, 51-631 Wrocław, Poland (e-mail: [email protected]) 2Museum of Natural History, Wrocław University, Sienkiewicza 21, 50-335 Wrocław, Poland (e-mail: [email protected]) *corresponding author abstract: Reproduction and growth of Vallonia pulchella (O. F. Müller) and V. costata (O. F. Müller) were studied in laboratory conditions. Their ellipsoid and singly laid eggs are among the smallest heavily calcified (mean size 0.68×0.67×0.52 and 0.69×0.67×0.54 mm in V. pulchella and V. costata, respectively). V. pulchella reached morphological maturity (lip completion) at 3.25–3.50 whorls (mean 3.35), 49 to 166 days after hatching (85). The first egg (sexual maturity) was laid at 3.25–3.50 whorls (3.35), 50–283 days after hatching (162). The life span ranged from 628 to 940 days (779), the time elapsing between the last egg and death was 12–184 days (111). In V. costata the lip was completed at 3.25–3.50 whorls (3.37), 42 to 183 days after hatching (120). The first egg was laid at 3.25–3.50 whorls (3.42), 131–290 days after hatching (193). -

(Mollusca) of the Slovak Republic
Vol. 15(2): 49–58 CHECKLIST OF THE MOLLUSCS (MOLLUSCA) OF THE SLOVAK REPUBLIC TOMÁŠ ÈEJKA*, LIBOR DVOØÁK, MICHAL HORSÁK, JOZEF ŠTEFFEK *Correspondence: Institute of Zoology, Slovak Academy of Sciences, Dúbravská cesta 9, SK-84506 Bratislava, Slovak Republic (e-mail: [email protected]) ABSTRACT: The checklist of 245 mollusc species known so far from the Slovak Republic is presented, plus 11 species limited to greenhouses or thermal waters. Critical comments on species erroneously mentioned in re- cent publications from Slovakia are included. KEY WORDS: Mollusca, checklist, Slovak Republic INTRODUCTION Research of Slovak molluscs started at the begin- cal evaluation of the previously published checklists ning of the 20th century (CSIKI 1918). In the first half (BANK et al. 2001, ŠTEFFEK &GREGO 2002). We deci- of the 20th century J. F. BABOR and later also his col- ded to use the monograph Molluscs of Slovakia (LI- league J. PETRBOK worked on the Slovak malaco- SICKÝ 1991) as the most suitable baseline because it fauna. Unfortunately their publications were not sys- contains the most recent reliable list of Slovak tematic and especially not critical enough, resulting molluscs. Therefore the original literature sources in erroneous records of some mollusc species in Slo- are given for all the species first recorded in the Slo- vakia (LISICKÝ 1991). The situation changed after vak Republic after 1982. World War II. The work of the new generation of The checklist of Slovak molluscs published by ŠTEF- malacologists resulted in a reliable knowledge about FEK &GREGO (2002) has several shortcomings. The the fauna. The entire research was dominated by the authors uncritically adopted many taxa from the work of V. -

High Population Differentiation in the Rock-Dwelling Land Snail (Trochulus Caelatus) Endemic to the Swiss Jura Mountains
Conserv Genet (2010) 11:1265–1271 DOI 10.1007/s10592-009-9956-3 RESEARCH ARTICLE High population differentiation in the rock-dwelling land snail (Trochulus caelatus) endemic to the Swiss Jura Mountains Sylvain Ursenbacher Æ Caren Alvarez Æ Georg F. J. Armbruster Æ Bruno Baur Received: 9 February 2009 / Accepted: 24 June 2009 / Published online: 14 July 2009 Ó Springer Science+Business Media B.V. 2009 Abstract Understanding patterns of genetic structure is Introduction fundamental for developing successful management pro- grammes for isolated populations of threatened species. The fragmentation of natural habitat is generally consid- Trochulus caelatus is a small terrestrial snail endemic to ered to be a major threat to many species. Population calcareous rock cliffs in the Northwestern Swiss Jura genetic theory predicts that the isolation of small popula- Mountains. Eight microsatellite loci were used to assess the tions lead to a reduction of genetic diversity. Human effect of habitat isolation on genetic population structure activities are often the main causes of habitat fragmenta- and gene flow among nine populations occurring on dis- tion, but geographical processes and/or specific habitat tinct cliffs. We found a high genetic differentiation among requirements may also contribute to natural segregation of populations (mean FST = 0.254) indicating that the popu- populations. Species with limited dispersal ability partic- lations are strongly isolated. Both allelic richness and ularly suffer from isolation, which may lead to a marked effective population size were positively correlated with genetic divergence among populations (e.g. Conner and the size of the cliffs. Our findings support the hypothesis Hartl 2004). -

Pulmonata, Helicoidea, Hygromiidae)
Ruthenica, 2019, vol. 29, No. 2: 77-86. © Ruthenica, 2019 Published online March 5, 2019 http: www.ruthenica.com On the phylogenetic relationships of Elbasania Schileyko et Fehér, 2017 (Pulmonata, Helicoidea, Hygromiidae) Marco T. NEIBER Universität Hamburg, Centrum für Naturkunde (CeNak), Zoologisches Museum, Abteilung Biodiversität der Tiere, Martin-Luther-King-Platz 3, 20146 Hamburg, GERMANY. E-Mail [email protected]; [email protected] ABSTRACT. The genus-group taxon Elbasania Schi- mainly on the basis of similarities of the dart appara- leyko et Fehér, 2017 has recently been introduced as a tus. subgenus of Metafruticicola Ihering, 1892 for a spe- In a comprehensive molecular phylogenetic study cies occurring in north-western Greece and Albania. Using mitochondrial and nuclear markers, the phyloge- of western Palearctic Helicoidea Rafinesque, 1815, netic relationships of Elbasania within Metafruticico- Razkin et al. [2015] classified the clade to which lini (Hygromiidae) are reconstructed. The results of hygromiids and related groups belong into three these analyses suggest that Elbasania is more closely newly delimited families: Canariellidae Schileyko, related to Hiltrudia Nordsieck, 1993, which has a range 1991, Geomitridae Boettger, 1909 and Hygromii- adjacent to that of Elbasania from Croatia to northern dae. The Hygromiidae were classified into three Albania, than to Metafruticicola. Elbasania shares subfamilies, Hygromiinae (including Trochulinae with Hiltrudia and also Cyrnotheba Germain, 1929 a Lindholm, 1927 and Monachainae Wenz, 1930 very characteristic microsculpture of the shell and an (1904)), Ciliellinae Schileyko, 1970 and Leptaxinae overall similar genital system, which however differs Boettger, 1909. However, the sampling of Hygromi- among these three taxa with regard to its internal struc- idae was focused on West European taxa and repre- tures, especially those of the penis. -

European Red List of Non-Marine Molluscs Annabelle Cuttelod, Mary Seddon and Eike Neubert
European Red List of Non-marine Molluscs Annabelle Cuttelod, Mary Seddon and Eike Neubert European Red List of Non-marine Molluscs Annabelle Cuttelod, Mary Seddon and Eike Neubert IUCN Global Species Programme IUCN Regional Office for Europe IUCN Species Survival Commission Published by the European Commission. This publication has been prepared by IUCN (International Union for Conservation of Nature) and the Natural History of Bern, Switzerland. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, the Natural History Museum of Bern or the European Union concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN, the Natural History Museum of Bern or the European Commission. Citation: Cuttelod, A., Seddon, M. and Neubert, E. 2011. European Red List of Non-marine Molluscs. Luxembourg: Publications Office of the European Union. Design & Layout by: Tasamim Design - www.tasamim.net Printed by: The Colchester Print Group, United Kingdom Picture credits on cover page: The rare “Hélice catalorzu” Tacheocampylaea acropachia acropachia is endemic to the southern half of Corsica and is considered as Endangered. Its populations are very scattered and poor in individuals. This picture was taken in the Forêt de Muracciole in Central Corsica, an occurrence which was known since the end of the 19th century, but was completely destroyed by a heavy man-made forest fire in 2000. -

Open Carboniferous Limestone Pavement Grike Microclimates in Great Britain and Ireland: Understanding the Present to Inform the Future
Open Carboniferous Limestone pavement grike microclimates in Great Britain and Ireland: understanding the present to inform the future Item Type Thesis or dissertation Authors York, Peter, J. Citation York, P, J. (2020). Open Carboniferous Limestone pavement grike microclimates in Great Britain and Ireland: understanding the present to inform the future (Doctoral dissertation). University of Chester, UK. Publisher University of Chester Rights Attribution-NonCommercial-NoDerivatives 4.0 International Download date 10/10/2021 01:26:52 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10034/623502 Open Carboniferous Limestone pavement grike microclimates in Great Britain and Ireland: understanding the present to inform the future Thesis submitted in accordance with the requirements of the University of Chester for the degree of Doctor of Philosophy By Peter James York April 2020 I II Abstract Limestone pavements are a distinctive and irreplaceable geodiversity feature, in which are found crevices known as grikes. These grikes provide a distinct microclimate conferring a more stable temperature, higher relative humidity, lower light intensity and lower air speed than can be found in the regional climate. This stability of microclimate has resulted in an equally distinctive community of flora and fauna, adapted to a forest floor but found in an often otherwise barren landscape. This thesis documents the long-term study of the properties of the limestone pavement grike in order to identify the extent to which the microclimate may sustain its distinctive biodiversity, to provide recommendations for future research which may lead to more effective management. Over a five-year study, recordings of temperature, relative humidity, light intensity and samples of invertebrate biodiversity were collected from five limestone pavements situated in the Yorkshire Dales and Cumbria in Great Britain, and The Burren in the Republic of Ireland. -

Hairy Snail Trochulus Hispidus (Linnaeus, 1758) in Flight – a Note on Avian Dispersal of Snails
Folia Malacol. 21(2): 111–112 http://dx.doi.org/10.12657/folmal.021.013 HAIRY SNAIL TROCHULUS HISPIDUS (LINNAEUS, 1758) IN FLIGHT – A NOTE ON AVIAN DISPERSAL OF SNAILS 1 2 STANIS£AW RUSIECKI , AGNIESZKA RUSIECKA 1Museum of Natural History, Wroc³aw University, Sienkiewicza 21, 50-335 Wroc³aw, Poland (e-mail: [email protected]) 2Department of Animal Physiology and Biostructure, Wroc³aw University of Environmental and Life Sciences, Norwida 31, 50-375 Wroc³aw, Poland ABSTRACT: A specimen of hairy snail Trochulus hispidus L. was found in the plumage of a great tit, Parus major, wintering in SW Poland. This passerine is the smallest bird species recorded carrying a gastropod. KEY WORDS: Trochulus hispidus, Trichia hispida, Parus major, dispersal, transport, snail, bird The idea of dispersal of snails (and molluscs in ter male great tit, P. major (identification according to general) by migratory birds was first proposed in the SVENSSON 1992). The bird had several feather cysts nineteenth century (DARWIN 1859, KEW 1893). Ob- (folliculoma) on the upper breast but was in good servations and experiments showed that snails could overall condition. The shell was found while taking be transported by birds in two main ways: internally, body measurements, a standard procedure used for in the host’s digestive tract (MALONE 1965a, CADÉE every individual caught. It was the only snail we found 1988, 2011, KAWAKAMI et al. 2008, ANDERS et al. 2009, on a bird while ringing in the winter seasons VAN LEEUWEN et al. 2012, WADA et al. 2012) or exter- 2007–2012 in the locality mentioned above, where nally, attached to the plumage or legs of water birds 854 great tits were caught (1,389 birds in total). -
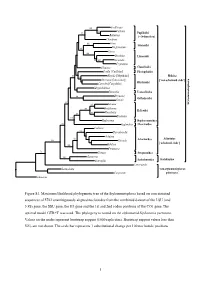
Figure S1. Maximum Likelihood Phylogenetic Tree of The
100 Cochlicopa 55 Vallonia 92 Pupilloidei Buliminus [= Orthurethra] Chondrina Arion 100 Arionoidei 66 Meghimatium Vitrina 100 Oxychilus Limacoidei 82 100 Euconulus Cryptozona Albinaria Clausilioidei Corilla [Corillidae] Plectopyloidea 70 Rhytida [Rhytididae] Helicina 53 Dorcasia [Dorcasiidae] [‘non-achatinoid clade’] Caryodes [Caryodidae] Rhytidoidei Megalobulimus Testacella Testacelloidea Drymaeus 94 Orthalicoidei Gaeotis 82 93 Satsuma Stylommatophora 100 Bradybaena Helicoidei Monadenia 87 93 84 Trochulus Haplotrema Haplotrematoidea 93 Euglandina Oleacinoidea Coeliaxis 92 Thyrophorella Achatina 92 Achatinina 100 Glessula Achatinoidea [‘achatinoid clade’] 100 Subulina Ferussacia 76 Gonaxis Streptaxoidea 100 Guestieria Systrophia Scolodontoidea Scolodontina Laevicaaulis Laemodonta ‘non-stylommatophoran Carychium pulmonates’ Siphonaria 1% 0.01 Figure S1. Maximum likelihood phylogenetic tree of the Stylommatophora based on concatenated sequences of 5782 unambiguously aligned nucleotides from the combined dataset of the LSU (and 5.8S) gene, the SSU gene, the H3 gene and the 1st and 2nd codon positions of the CO1 gene. The optimal model GTR+G was used. The phylogeny is rooted on the siphonariid Siphonaria pectinata. Values on the nodes represent bootstrap support (1000 replicates). Bootstrap support values less than 50% are not shown. The scale bar represents 1 substitutional change per 100 nucleotide positions. 1 91 Satsuma 100 Bradybaena Trochulus 97 Helicoidei 68 Monadenia 87 Haplotrema Haplotrematoidea Euglandina Oleacinoidea 100 Vallonia