Physicochemical Quality of Borehole Water in Abonnema and Its Public Health Importance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Downloaded for Personal Non-Commercial Research Or Study, Without Prior Permission Or Charge
https://theses.gla.ac.uk/ Theses Digitisation: https://www.gla.ac.uk/myglasgow/research/enlighten/theses/digitisation/ This is a digitised version of the original print thesis. Copyright and moral rights for this work are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This work cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Enlighten: Theses https://theses.gla.ac.uk/ [email protected] THE POLITICS AMO ADMINISTRATION OF COhTUNITY DEVELOPMENT IN THE RIVERS STATE OF NIGERIA BY LAURENCE A.8. lYAGOA Submitbed for the Degree of Doctor of Philosophy University of Glasgow Duly 1976 ProQuest Number: 10647271 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uesL ProQuest 10647271 Published by ProQuest LLO (2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code Microform Edition © ProQuest LLO. ProQuest LLO. -

The Challenges of Developing Buguma Town As a Secondary City in Rivers State Nigeria
KIU Journal of Social Sciences KIU Journal of Social Sciences Copyright©2019 Kampala International University ISSN: 2413-9580; 5(2): 19–30 The Challenges of Developing Buguma Town as a Secondary City in Rivers State Nigeria VICTOR OWITUBO CHARLES, NALUBA GOODY NWIEKPIGI Ignatius Ajuru University of Education, Rumuolumeni, Port Harcourt Abstract. This study investigated the challenges Keywords: Challenges, Secondary, City, of developing Buguma as a secondary city in Developing. Rivers State. This study made use of public opinion survey research design with a sample 1. Introduction size of 200 respondents. Data was sourced from the research instrument known as developing 1.1 Background and Overview Buguma Secondary City Questionnaire (DBSCQ) designed along the Likert-type 4-point The philosophy underlying regional planning scale rating. Data was analyzed with both programmes is that the problems of urban and descriptive and inferential statistics. Simple rural areas are intricately connected and should percentage was used to analyze the descriptive be so solved within a regional framework and inferential statistics. Simple percentage was (Slatter 2012, World Bank, 2011). Similarly, used to analyze the socio-demographic global economic activities and population background of the respondents while mean and dynamics are increasingly concentrated in big standard deviation were used to analyze the cities and as a result, urban research has tended research questions. One way analysis of variance to focus on large metropolitan areas at the (ANOVA) was used to analyze the hypotheses. expense of secondary cities which have been a The results of the study revealed that majority of rediscovery of the city in academic discourse the respondents were male 108 (54%); were (Marais, Rooyen, Lenka and Cloete. -

Assessment of Soil Quality in Representative Pedons of the Sombreiro Warri Deltaic Plain of the Niger Delta, Nigeria
International Journal of Agriculture and Earth Science Vol. 3 No. 5 ISSN 2489-0081 2017 www.iiardpub.org Assessment of Soil Quality in Representative Pedons of the Sombreiro Warri Deltaic Plain of the Niger Delta, Nigeria Kamalu1* O. J., Udom1 B. E. & Omenihu2 A. A. 1Crop and Soil Science Department, University of Port Harcourt 2Soil Science Department, Abia State University Umudike-Umuahia Campus Abia State 1*[email protected] ABSTRACT Assessment of soil quality of the Ogba-Egbema area of the Sombreiro Warri Deltaic Plain was undertaken by morphological characterization and ranking of soil physical and chemical properties. A total of 80 genetic soil samples from 16 soil profiles and one hundred and twenty (120) random surface samples taken from farm plots along eight (8) transects in the area were studied. The ranking of the eight transects in a descending order was: Akabuka/Obite > Umuoru/Ndoni > Omoku/Egbegoro > Obagi/Ogbogu > Obiafu > Aggah/Egbema > Ebocha/Okwuzi > Omoku/Elele. From the results obtained the top three ranked transects: Akabuka/Obite, Umuoru/Ndoni and Omoku/Egbegoro were rated 59.0, 52.0 and 42.7% respectively of the various parameters qualifying for high fertility indices while the three transects with lowest quality (Aggah/Egbema, Ebocha/Okwuzi and Omoku/Elele) had only 28.8, 22.7 and 21.0% respectively of sample stations that qualified them for high soil quality status. Five soil properties: drainage, depth, total organic matter, soil colour and texture, were delineated as the minimum data set needed for soil quality determination in the area. Most of the fertility indices were below critical limits as expressed by low ECEC, low organic matter and low available P. -

Draft Environmental and Social Impact Assessment (Esia) Report for the Proposed Bonny Deep Sea Port Project in Bonny Island
DRAFT ENVIRONMENTAL AND SOCIAL IMPACT ASSESSMENT (ESIA) REPORT FOR THE PROPOSED BONNY DEEP SEA PORT PROJECT IN BONNY ISLAND, BONNY LOCAL GOVERNMENT AREA, RIVERS STATE BY FEDERAL MINISTRY OF TRANSPORTATION SUBMITTED TO THE FEDERAL MINISTRY OF ENVIRONMENT HEADQUARTERS MABUSHI, ABUJA DECEMBER, 2020 DRAFT ENVIRONMENTAL and social impact ASSESSMENT (esia) REPORT FOR THE PROPOSED BONNY DEEP SEA PORT PROJECT IN BONNY ISLAND, BONNY LOCAL GOVERNMENT AREA, RIVERS STATE PREPARED BY ESCHOLES INTERGRATED SERVICES RESOURCES LIMITED NAME FUNCTION SIGNATURE 1st Reviewed by Dr. Eseoghene okereka 2nd reviewed Kelvin Olisameka by QA/QC L. A. MORAKINYO Compiled EZEH CHINENYE AYO LAWAL SOPHIA OGE STATUS 01 - DRAFT - December DRAFT ESIA REPORT OF THE PROPOSED BONNY DEEP SEA PORT PROJECT AT BONNY L.G.A, RIVERS STATE BY FEDERAL MINISTRY OF TRANSPORTATION TABLE OF CONTENT List of Tables List of Figures List of Plates List of Abbreviation and Acronyms ESIA preparers Acknowledgement Executive Summary CHAPTER ONE: INTRODUCTION 1.1 Background Information 1-1 1.2 Project Proponent 1-2 1.3 Objectives of the ESIA 1-3 1.4 Terms of Reference for the Study 1-4 1.5 Administrative and Legal Framework 1-6 1.5.1National Regulations 1-6 1.5.1.1 Environmental Impact Assessment Act 86 of 1992 1-8 1.5.1.2 Environmental Impact Assessment Sectoral Guidelines for 1-10 Infrastructures 1995 1.5.1.3 National Policy on Environment 2017 1-10 1.5.1.4 National Guidelines and Standard for Water Quality 1999 1-11 1.5.1.5National Guidelines on Environmental Management Systems 1-11 (EMS) 1999 -

World Bank Document
SFG2386 V4 Public Disclosure Authorized ENVIRONMENTAL AND SOCIAL MANAGEMENT PLAN (ESMP) Draft Report For PUBLIC WORKS – Road Maintenance Public Disclosure Authorized (90 Lots) in Twenty-three Local Government Areas, Rivers State Public Disclosure Authorized By Public Disclosure Authorized Rivers State Employment and Expenditure for Results Project (SEEFOR), Ministry of Budget & Economic Planning Under the World Bank Assisted Project and European Union July, 2017 SEEFOR Intervention Works, Road Maintenance, Rivers State Table of Contents Content Page Title Cover i Table of Contents ii List of Tables iii List of Acronyms iv Executive Summary v CHAPTER ONE: Introduction 1 1.1 Background 1 1.2 The Project Invention Locations 1 1.3 Description of Proposed Activities 1 1.4 Need for ESMP 2 1.5 Approaches for Preparing the ESMP 2 1.5.1 Literature Review 2 1.5.2 Field Visits 2 CHAPTER TWO: Institutional and Legal Framework for Environmental Management 3 2.1 Introduction 3 2.2 Federal Policy/Legislation 3 2.2.1 National Policy on Environment 3 2.2.2 Federal EIA Act No. 86, 1992 3 2.3 Rivers State Environment Protection Law No. 2, 1994 3 2.4 World Bank Safeguards Policy Triggered by SEEFOR & the Proposed Project 3 CHAPTER THREE: Biophysical Environment 4 3.1 Introduction 4 3.2 Project Location 4 3.3 Geology and Ecological Zones 4 3.4 Climate and Rainfall 4 3.5 Waste and Waste Management 4 3.6 Air Quality and Noise Level 4 3.7 Population of the Intervention Areas 4 3.8 Economy and Source of Livelihood 4 3.9 Culture of the People ` 4 3.10 Social Structure -

Internal Communication Clearance Form
NATIONS UNIES UNITED NATIONS HAUT COMMISSARIAT DES NATIONS UNIES OFFICE OF THE UNITED NATIONS AUX DROITS DE L’HOMME HIGH COMMISSIONER FOR HUMAN RIGHTS PROCEDURES SPECIALES DU SPECIAL PROCEDURES OF THE CONSEIL DES DROITS DE L’HOMME HUMAN RIGHTS COUNCIL Mandates of the Special Rapporteur on adequate housing as a component of the right to an adequate standard of living, and on the right to non-discrimination in this contextand of the Special Rapporteur on extreme poverty and human rights Téléfax: (41-22) 917 9006 Address: Télégrammes: UNATIONS, GENEVE Palais des Nations Téléx: 41 29 62 CH-1211 GENEVE 10 Téléphone: (41-22) 917 9359 Internet www.ohchr.org E-mail: [email protected] REFERENCE: AL Housing (2000-9) Poverty (1998-11) NGA 2/2012 25 July 2012 Excellency, We have the honour to address you in our respective capacities as Special Rapporteur on adequate housing as a component of the right to an adequate standard of living, and on the right to non-discrimination in this context and as Special Rapporteur on extreme poverty and human rights, pursuant to Human Rights Council resolutions 15/8 and 17/13. In this connection, we would like to bring to your Excellency’s Government’s attention information we have received concerning the alleged eviction and demolition of the Abonnema Wharf Community, in Port Harcourt, Nigeria. According to the information received: On 27 June 2012, at approximately 07:00 a.m., an estimated 10,000 to 20,000 residents of the Abonnema Wharf Community in Port Harcourt, capital of Rivers State, located in Nigeria’s Niger Delta, were evicted and their homes and small businesses demolished. -

'Just Move Them' Forced Evictions in Port Harcourt, Nigeria Contents
‘just move them’ FORCED EVICTIONS IN PORT HARCOURT, NIGERIA housing is a human right amnesty international is a global movement of 2.8 million supporters, members and activists in more than 150 countries and territories who campaign to end grave abuses of human rights. our vision is for every person to enjoy all the rights enshrined in the universal declaration of human rights and other international human rights standards. We are independent of any government, political ideology, economic interest or religion and are funded mainly by our membership and public donations. amnesty international publications first published in 2010 by amnesty international publications international secretariat peter Benenson house 1 easton street London Wc1X 0dW united Kingdom www.amnesty.org © amnesty international publications 2010 index: afr 44/017/2010 original language: english printed by amnesty international, international secretariat, united Kingdom all rights reserved. this publication is copyright, but may be reproduced by any method without fee for advocacy, campaigning and teaching purposes, but not for resale. the copyright holders request that all such use be registered with them for impact assessment purposes. for copying in any other circumstances, or for reuse in other publications, or for translation or adaptation, prior written permission must be obtained from the publishers, and a fee may be payable. to request permission, or for any other inquiries, please contact [email protected] Cover photo : abonnema Wharf waterfront in port harcourt, rivers state, nigeria. the area has been named as the next to be demolished as part of the city’s redevelopment programme. thousands of residents are at risk of forced eviction as a result. -

Perception and Rating of the Environmental Quality of Bonny
IJRES 8 (2021) 14-26 ISSN 2059-1977 Perception and rating of the environmental quality of doi.org/10.33500/ Bonny Ancient Town ijres.2021.08.002 Ogoro Mark1*, Furo-Vincent Evelyn2 and Dollah Osademe Chukwudi3 1Department of Geography and Environmental Management, University of Port Harcourt, Nigeria. 2Institute of Natural Resources, Environment and Sustainable Development, University of Port Harcourt, Nigeria. 3Institute of Geosciences and Space Technology (IGST), Rivers State University, Nkpolu-Oroworukwo, Port Harcourt, Nigeria. Article History ABSTRACT Received 16 February, 2020 Urban environmental quality has a lot to do with city livability, quality of life and Received in revised form 11 sustainability. The well-being, health care and general condition of urban cities May, 2020 Accepted 14 May, 2020 have been of major concern in recent years. This work seeks evaluated and rated the environmental quality of Bonny Ancient Town. Questionnaire analysis was Keywords: employed to retrieve information on individual perception of the environment. The Environment, global positioning system was used to extract the X and Y coordinates of each Quality, household as sampled. Finding reveals that the region enjoys slightly too high Urban, environmental quality across locations on the eastern part of Bonny, and also Density. residential density influences the environmental quality as observed. It is therefore recommended that an extensive baseline studies should be conducted across Bonny local government area (LGA). And also a well-coordinated and Article Type: integrated environmental management practice should be put in place in order to Full Length Research Article curb area with noticed poor environmental qualities. ©2021 Blue Pen Journals Ltd. -

Port Harcourt Fact Finding Mission
EVICTIONS AND DEMOLITIONS IN PORT HARCOURT REPORT OF FACT-FINDING MISSION TO PORT HARCOURT CITY, FEDERAL REPUBLIC OF NIGERIA 12-16 MARCH 2009 UN-HABITAT with Ministry of Works, Housing and Urban Development of the Federal Republic of Nigeria, Social and Economic Rights Action Center (SERAC), Women Environment Programme (WEP) The responsibility for the content of this report, including its recommendations, rests entirely with UN-HABITAT. The time devoted to the Mission and the contributions of those individuals and organisations listed in the annexes are acknowledged and appreciated. This report has been produced without formal editing by the United Nations. The designations employed and the presentation of the material in this report do not imply the expression of any opinion whatsoever on the part of the United Nations Secretariat concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Reference to names of firms and commercial products and processes does not imply their endorsement by the United Nations, and a failure to mention a particular firm, commercial product or process is not a sign of disapproval. Excerpts from the text may be reproduced without authorization, on condition that the source is indicated. Cover photo: Legal structure along the upper part of Abonnema Wharf, demolished during the period 10 to 13 February 2009. Photo credit: Social and Economic Rights Action Center (SERAC) UN-HABITAT Abuja and Nairobi, August 2009 ii -

Alafaka Tobin M.B, B.S
A COMPARISON OF UPTAKE OF INTERMITTENT PREVENTIVE TREATMENT OF MALARIA IN PREGNANCY IN URBAN AND RURAL PUBLIC PRIMARY HEALTH CARE CENTRES IN RIVERS STATE BY ALAFAKA TOBIN M.B, B.S A DISSERTATION SUBMITTED TO THE NATIONAL POSTGRADUATE MEDICAL COLLEGE OF NIGERIA IN PART FULFILLMENT OF THE REQUIREMENTS FOR THE AWARD OF FELLOWSHIP OF THE MEDICAL COLLEGE IN PUBLIC HEALTH MAY, 2016 1 DECLARATION I hereby declare that this study, or part of it, has not been and will not be submitted for any other diploma, fellowship or any other examination. ……………..………. ALAFAKA TOBIN M.B, B.S 2 CERTIFICATION This is to certify that this dissertation titled “A Comparison Of Uptake Of Intermittent Preventive Treatment Of Malaria In Pregnancy In Urban And Rural Public Primary Health Care Centres In Rivers State” was carried out by Alafaka Tobin, a Senior Registrar in the Department of Community Medicine, University of Port Harcourt Teaching Hospital under our supervision. ………………………….. ………………..…… Dr. C. I. Tobin-West Dr. C. A. Onoka MD, MPH, FMCPH MBBS, DLSHTM, MPH, MSc, FWACP Senior Lecturer, Consultant Community Medicine Lecturer, Consultant Community Medicine Department of Community Medicine University of Nigeria Teaching Hospital University of Port Harcourt Teaching Hospital Nsukka, Enugu Port Harcourt, Rivers State …………………… Dr. K. E. Douglas Head of Department Community Medicine University of Port Harcourt Teaching Hospital Port Harcourt Rivers State 3 DEDICATION This book is dedicated to my husband Mr. Derefaka Tobin whose words of encouragement motivated me to commence and complete this dissertation. 4 ACKNOWLEDGEMENT I am very grateful to my supervisors, Dr. C.I. Tobin-West and Dr. -
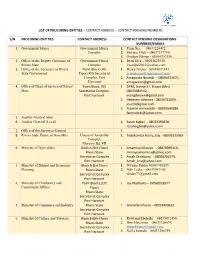
List of Procuring Entities – Contact Address – Contact Persons/Numbers
LIST OF PROCURING ENTITIES – CONTACT ADDRESS – CONTACT PERSONS/NUMBERS S/N PROCURING ENTITIES CONTACT ADDRESS CONTACT PERSONS-DESIGNATIONS- NUMBERS/EMAILS 1. Government House Government House 1. Perm Sec. – 08037224492 Complex 2. Margret Dick – 08037277770, 3. Oyoboy Edman - 08036757330 2. Office of the Deputy Governor of Government House 1. Iwari Jack – 08055625938, Rivers State Complex [email protected] 3. Office of the Secretary to Rivers Point Block (4th 1. Henry Nzeka – 08038831847, State Government Floor),R/S Secretariat [email protected] Complex, Port 2. Amagwula Nnamdi – 08064525671, Harcourt [email protected] 4. Office of Head of Service of Rivers Point Block, R/S 1 DPRS, Inyingi S.I. Brown (Mrs) - State Secretariat Complex, 08033384942, Port Harcourt [email protected] 2 Hekerem Adanma - 08036721069, [email protected] 3 Franklin Aminadoki – 08035640289 [email protected] 5. Auditor -General State 6. Auditor General (Local) 1. Sarah Kigbel - 08033393878 [email protected] 7. Office of the Surveyor-General 1. 8. Rivers State House of Assembly House of Assembly 1. Tubokomba Harry, Esq. - 08033125065 Complex Moscow Rd, PH 9. Ministry of Agriculture Block A (5th Floor) 1. Amamina Minaiyo - 08078969110, Rivers State [email protected] Secretariat Complex 2. Amah Christiana - 08036702579, Port Harcourt [email protected] 3. Ministry of Budget and Economic Block B (1st Floor) 1. Nwume Friday 08039382679 Planning Rivers State 2. Ndii Ledee - 08033097140 Secretariat Complex [email protected] Port Harcourt 4. Ministry of Chieftaincy and Point Block (11th 1. Joy Wariboko – 07068018977 Community Affairs Floor) Rivers State Secretariat Complex Port Harcourt 5. Ministry of Commerce and Industry Rivers State 1. Granville Elfreda – 08033409613 Secretariat Complex Port Harcourt 6. -

World Rural Observations 2019;11(3) WRO 11 an Appraisal of the Role of Community Development
World Rural Observations 2019;11(3) http://www.sciencepub.net/rural WRO An Appraisal of the Role of Community Development Committees (CDCs) in Rural Communities Sokeipirim Godstime Douglas1 and Sampson Thompson Ekpo2 1Department of Urban and Regional Planning, School of Environmental Sciences, Captain Elechi Amadi Polytechnic, Rumuola, P.M.B. 5936, Port Harcourt, Rivers State, Nigeria 2Centre for Children with Development and Communication Disorders. University of Port Harcourt, P.M.B. 5323, Choba, Port Harcourt, Rivers State, Nigeria Tel: +2347034543973; [email protected] Abstract: Rural development is a process whereby people who reside and work outside the urban areas are made to function without lowering their honour or quality of living. Activities and persons who are to make this possible must be seen to be determined, transparent and knowledgeable. Community Development Committees (CDCs) should be prepared in all ramifications to bring about rural development. There are functions lined out for them which many CDCs seem not to know or have abandoned. The chiefs should help their communities to make peace. Members of CDCs should have the interest of their community at heart as it is seen to be happening at Abonnerna. This paper critically examined the role of CDCs and also observed works they do. It is important that accountability should be taken seriously by the members; while proper monitoring and funding by the Local Government Areas. [Douglas, S. G. and Ekpo, S. T. An Appraisal of the Role of Community Development Committees (CDCs) in Rural Communities. World Rural Observ 2019;11(3):11-14]. ISSN: 1944-6543 (Print); ISSN: 1944-6551 (Online).