8440 Medical Multiple
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HMCS Estate by Region & Estates
Her Majesty’s Courts Service Estate by Region and Area London Region (Civil and Family) London Region (Crime) v Barnet Enfield v Edmonton L Barnet Wood Green L Romford Harrow Hendon Haringey G3 Waltham Forest Havering L v v v Snaresbrook v G3 v Highbury Corner Ilford G3 Redbridge Clerkenwell & Shoreditch Brent v Thames Willesden L Berwick upon Tweed v v L (Gee Street) Bow v Barking Uxbridge PRFD L L v Uxbridge L v RCJ Stratford v West London Central London S v L L RCJ Mayor's & City of London Ealing Marylebone IL&CFP Central Criminal Court v L Acton v v G2 vCity of London v v Middlesex Guildhall v IL&CFP Woolwich v G3 GSouthwark Woolwich Brentford Brentford 3 L Isleworth Horseferry Rd.v G3 vTower Bridge G3 v L Lambeth G v v Blackfriars G3 L 3 West London Inner London Sessions House Wandsworth Richmond Upon Thames South Western v v L v v Camberwell Green Greenwich Bexley Hounslow South West Balham v v Alnwick v Wimbledon Kingston Upon Thames North West Region Kingston Upon Thames v Bromley v Bromley L L G3 v v Croydon Croydon L Sutton G3 v v Morpeth L Bedlington v Gosforth v Hexham Newcastle upon Tyne v Blaydonv I1 v Lv North Shields Carlisle GatesheadLv LvSouth Shields I1 v Chester Le Street LvSunderland ConsettLv v vHoughton Le Spring Durham Peterlee G3 Lv Bishop Auckland v Symbol Legend Lv Hartlepool Workington Penrith Newton Aycliffe Lv v Lv v North East Region G Crown Court Darlington Whitehaven Lv Teesside First, Second, Third Guisborough G1 G2 G3 Lv I1 v v Whitby I Combined Court Centre v I1 First, I 2 Second, I 3 Third Kendal Lv -

WOOLWICH and ELTHAM SUNDAY FOOTBALL ALLIANCE Founded 2006
WOOLWICH AND ELTHAM SUNDAY FOOTBALL ALLIANCE Founded 2006 APPLICATION FORM INFORMATION 2016-2017 INCORPORATING THE PLUMSTEAD CHALLENGE CUP THIS ALLIANCE WAS FORMED BY THE WOOLWICH AND DISTRICT SUNDAY FOOTBALL LEAGUE (FORMED 1891) AND THE ELTHAM AND DISTRICT SUNDAY FOOTBALL LEAGUE (FORMED 1959) AFFILIATED TO THE LONDON FOOTBALL ASSOCIATION WOOLWICH AND ELTHAM SUNDAY FOOTBALL ALLIANCE Founded 2006 (INCORPORATING THE PLUMSTEAD CHALLENGE CUP) Unless stated, all correspondence should be addressed to the Hon. League Secretary Hon. Chairman Mr Shayne Hoadley 07985 807174 [email protected] Hon. Deputy Chairman Dear Applicant, Mr Dave Fone 07957 376392 [email protected] APPLICATION FOR MEMBERSHIP Hon. League Secretary Mr Jason Verrillo 07795 956379 [email protected] We refer to your recent enquiry. Hon. Treasurer Mrs Steph Pinner 07951 219531 In this downloaded document you will find the application “pack” information and [email protected] instructions. Hon. Referees’ & Interim Fixtures Secretary Mr David Hooker Please ensure that where possible, ALL questions are completed. Please note that 07766 541061 [email protected] or we are a Sunday morning football league and therefore our kick off times are [email protected] 10:30am apart from Cup Finals and the occasional fixture. Hon. Assistant Referees’ Secretary Mr Stuart Axford 07958 287924 Upon the completion and return of this application form you will be contacted and [email protected] advised to attend an interview with the Management Committee. Details of the date, Hon. Registration Secretary Mrs Kelly Hooker time and location will be provided. 07722 271194 [email protected] Hon. Results Secretary Please ensure you bring with you the £20.00 application fee on the day of interview Ms Lisa Brooks or post it along with this form to the League Secretary. -
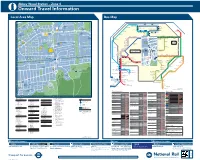
Abbey Wood Station – Zone 4 I Onward Travel Information Local Area Map Bus Map
Abbey Wood Station – Zone 4 i Onward Travel Information Local Area Map Bus Map 45 1 HARTSLOCK DRIVE TICKFORD CLOSE Y 1 GROVEBURY ROAD OAD 16 A ALK 25 River Thames 59 W AMPLEFORTH R AMPLEFORTH ROAD 16 Southmere Central Way S T. K A Crossway R 1 B I N S E Y W STANBROOK ROAD TAVY BRIDGE Linton Mead Primary School Hoveton Road O Village A B B E Y W 12 Footbridge T H E R I N E S N SEACOURT ROAD M E R E R O A D M I C H A E L’ S CLOSE A S T. AY ST. MARTINS CLOSE 1 127 SEWELL ROAD 1 15 Abbey 177 229 401 B11 MOUNTJOYCLOSE M Southmere Wood Park ROAD Steps Pumping GrGroroovoveburyryy RRoaadd Willow Bank Thamesmead Primary School Crossway Station W 1 Town Centre River Thames PANFIE 15 Central Way ANDW Nickelby Close 165 ST. HELENS ROAD CLO 113 O 99 18 Watersmeet Place 51 S ELL D R I V E Bentham Road E GODSTOW ROAD R S O U T H M E R E L D R O A 140 100 Crossway R Gallions Reach Health Centre 1 25 48 Emmanuel Baptist Manordene Road 79 STANBROOK ROAD 111 Abbey Wood A D Surgery 33 Church Bentham Road THAMESMEAD H Lakeside Crossway 165 1 Health Centre Footbridge Hawksmoor School 180 20 Lister Walk Abbey Y GODSTOW ROAD Footbridge N1 Belvedere BUR AY Central Way Wood Park OVE GROVEBURY ROAD Footbridge Y A R N T O N W Y GR ROAD A Industrial Area 242 Footbridge R Grasshaven Way Y A R N T O N W AY N 149 8 T Bentham Road Thamesmead 38 O EYNSHAM DRIVE Games N Southwood Road Bentham Road Crossway Crossway Court 109 W Poplar Place Curlew Close PANFIELD ROAD Limestone A Carlyle Road 73 Pet Aid Centre W O LV E R C O T E R O A D Y 78 7 21 Community 36 Bentham Road -

Directory of Services
Directory of Services Substance Misuse support for Residents of the Royal Borough of Greenwich Opiates and Alcohol support for dependent users CRi Aspire Service Support available: Day programme, one to one support, access to prescribing, 821 Woolwich Road, group work, access to community detox, referrals for residential treatment, key working, psychology support, peer mentoring, workshops, recovery community, Charlton SE7 8JL complimentary therapies and pre detox group. Open access drop in available every Tel: 020 8316 0116 day between 10 am - 12pm Individuals with complex support needs will be referred onto the specialist service at The Beresford Project. Cannabis, cocaine and other drugs - Non dependent users Lifeline BaSIS project Support available: Brief advice and information, motivational interviewing, goal Royal Arsenal Medical Centre setting, healthy living tips, self management techniques, acupuncture, group work. 21 Arsenal Way , Woolwich Brief support available for between one to twelve weeks dependent on need. London SE18 6TE Support for users of Cocaine, MDMA, Cannabis, Ketamine, Legal Highs, Methadrone, other Club Drugs and Alcohol. Open access drop in available 9am and 4:30pm Mon Tel: 020 3696 2640 - Fri and 9.30am - 12.30pm Sat Email: [email protected] Older Drinkers - over 55s Silver Lining Project Community and therapeutic support, key working, one to one support, group work, peer mentoring. Home visits (where appropriate and available). Referrals to other 2-6 Sherard Road, services including CRi Aspire -for community detox and access to residential Eltham, treatment. London SE9 Please call to make a referral or an appointment Tel: 079020 876 983 Email: [email protected] Family and Carer support Specialist family support workers from ADFAM are available within our Aspire ADFAM services. -

Gloucester County COVID-19 Cases Case # DATE SEX AGE Town/Municipality 26109 5/4/21 Female 40 Swedesboro 26108 5/4/21 Male 55 Greenwich Twp
NEWS For Immediate Release: Tuesday, May 4, 2021 Contact: Commissioner Director Robert M. Damminger (856-853-3395) Gloucester County Health Officer Annmarie Ruiz (856-218-4131) Gloucester County updates on COVID-19 As of Tuesday, May 4, 2021, Gloucester County has conducted 176,874 total tests. Of these cases, 150,765 have come back negative. Today, Gloucester County has an additional 79 cases to report. Gloucester County’s total positive COVID-19 case count is now 26,109. Gloucester County is reporting 581 deaths. The Gloucester County Board of Commissioners would like to remind residents that COVID-19 Vaccinations are readily available for all individuals living, working or studying in the State of New Jersey. Interested individuals can visit the Gloucester County Mega Site at Rowan College of South Jersey, 1400 Tanyard Rd. Sewell, NJ 08080. “The Mega Site will be operating with a revised schedule through May due to holidays and to allow Rowan College of South Jersey to begin renovations. The entire schedule can be found on our website,” said Commissioner Director Robert M. Damminger. “This will not hinder our ability to serve any individual who is looking to receive the COVID-19 vaccination.” Director Damminger shared that to date, the Gloucester County Mega Vaccination Site has vaccinated 385,211 individuals. COVID-19 testing is available weekdays, you must call (856) 218-0300 to be prescreened by Rowan Medicine and scheduled. A full list of today’s reported cases and deaths, including age, sex and municipality, is available in the charts below. Gloucester County COVID-19 Cases Case # DATE SEX AGE Town/Municipality 26109 5/4/21 Female 40 Swedesboro 26108 5/4/21 Male 55 Greenwich Twp. -

122 Bus Time Schedule & Line Route
122 bus time schedule & line map 122 Crystal Palace - Plumstead, Bus Garage View In Website Mode The 122 bus line (Crystal Palace - Plumstead, Bus Garage) has 2 routes. For regular weekdays, their operation hours are: (1) Crystal Palace: 12:05 AM - 11:50 PM (2) Plumstead, Bus Garage: 12:01 AM - 11:46 PM Use the Moovit App to ƒnd the closest 122 bus station near you and ƒnd out when is the next 122 bus arriving. Direction: Crystal Palace 122 bus Time Schedule 64 stops Crystal Palace Route Timetable: VIEW LINE SCHEDULE Sunday 12:05 AM - 11:50 PM Monday 12:05 AM - 11:50 PM Plumstead Road / Plumstead Station (WM) Foreland Street, London Tuesday 12:05 AM - 11:50 PM Greenwich Community College (WP) Wednesday 12:05 AM - 11:50 PM Plumstead Road, London Thursday 12:05 AM - 11:50 PM Maxey Road (W) Friday 12:05 AM - 11:50 PM Plumstead Road / Woolwich Public Market (Z) Saturday 12:05 AM - 11:50 PM 4 Victory Parade, London Woolwich Arsenal Station (J) Claydown Mews (E) 122 bus Info Claydown Mews, London Direction: Crystal Palace Stops: 64 Gunner Lane (F) Trip Duration: 65 min Gunner Lane, London Line Summary: Plumstead Road / Plumstead Station (WM), Greenwich Community College (WP), Nightingale Place (G) Maxey Road (W), Plumstead Road / Woolwich Public Market (Z), Woolwich Arsenal Station (J), Claydown Woolwich Common (H) Mews (E), Gunner Lane (F), Nightingale Place (G), Woolwich Common (H), Royal Garrison Church (J), Royal Garrison Church (J) Well Hall Road / Shooters Hill Road (WR), Well Hall Road / Broad Walk (WS), Well Hall Road / Dunblane Well -

Annual Town Report 2013-2014
255th Annual Report for the Town of WOOLWICH MAINE For the Fiscal Year 2013-2014 TOWN OF WOOLWICH Annual Report of the Town Officers of the Town of Woolwich Maine For the fiscal year July 1, 2013– June 30, 2014 Lincoln County Publishing Co. Newcastle / Damariscotta, Me. ANNUAL REPORT Town Information Woolwich, Maine -Incorporated October 20, 1759 -Population: 3,072 (2010 Census) -Government: Annual Town Meeting, five member Board of Selectmen and Town Administrator -School: Woolwich Central School (K-8); Principal, Thomas M. Soule -Post Office: Woolwich Post Office 04579; Tel. 443-2000 Bath Post Office 04530; Tel. 443-9779 -Woolwich Historical Society, 21 Nequasset Road; 443-4833; Open June, July and August on Saturdays, 10 a.m.-4 p.m. and by appointment. Call Debbie Locke at 443-5684. -Cable Television - COMCAST Questions about your bill - 1 (207) 729-6663 Local Cable - Channel 3 - To put items on the local cable, please call the Town Office at 442-7094 -Solid Waste Disposal - Pine Tree Waste, Inc. - 442-7141 -Telephone Service: Fairpoint 442/443/386/882 Exchanges -Cemeteries: Nequasset Cemetery, Partridge Cemetery, Riverside Cemetery, Bailey Cemetery, Gould Cemetery, Grover Cemetery, Murphy’s Corner Cemetery, Laurel Grove Cemetery, Thwing’s Point Cemetery TOWN OF WOOLWICH -Town Office: Municipal Building, 13 Nequasset Road Selectmen’s Office, Town Administrator and Town Treasurer 442-7094 Tax Collector and Town Clerk 442-8723 Fax machine 442-8859 Animal Control Officer 737-2093 Shellfish Warden 371-2732 Office Hours of the Town Clerk and Tax Collector Monday 9 a.m.-5 p.m. Wednesday 9 a.m.-6 p.m. -

Death in Kingston Upon Thames”: Analysis of the Bonner Hill Cemetery Burial Records 1855-1911
Christopher French, “Death in Kingston upon Thames”: Analysis of the Bonner Hill Cemetery Burial records 1855-1911. (November, 2001) Introduction The last few years have seen a great deal of research into the whole area of mortality in the nineteenth century, tackling such questions as age-specific mortality, infant mortality, regional variations in mortality, and the impact of medical, environmental and general social conditions on general mortality levels.1 A major source for this research has been the Annual Reports and Decennial Supplements published by the Registrar General. These provide aggregate mortality data derived from the information provided on death registration certificates at four levels of locality: registration sub-districts, districts, counties and divisions.2 Besides this aggregate data, information on the death of individuals is contained in the Civil Registers, but since these are not available for historical research, the historian has to look elsewhere for sources which record information on individuals. If available – and comprehensive enough – such local sources can not only be used to help test conclusions reached from an analysis of the established national sources, but can also help highlight local variations in, for example, the mortality decline of the late nineteenth century and the reasons for this, and in infant mortality.3 Such a local source is the burial records held in municipal cemeteries. These are not as comprehensive as individual death certificates – cause of death is not recorded, for example. -

Written Guide
Trains and boats and planes A self guided walk around the riverside and docks at North Woolwich Discover how a remote marsh became a gateway to the world Find out how waterways have influenced economic boom, decline and revival See how various transport networks have helped to transform the area Explore a landscape rapidly evolving through regeneration .discoveringbritain www .org ies of our land the stor scapes throug discovered h walks 2 Contents Introduction 4 Route overview 5 Practical information 6 Detailed route maps 8 Commentary 10 Further information 33 Credits 34 © The Royal Geographical Society with the Institute of British Geographers, London, 2014 Discovering Britain is a project of the Royal Geographical Society (with IBG) The digital and print maps used for Discovering Britain are licensed to the RGS-IBG from Ordnance Survey Cover image: University of East London campus buildings © Rory Walsh 3 Trains and boats and planes Explore the changing riverside and docks at North Woolwich For centuries the part of East London now known as North Woolwich was a remote marsh by the River Thames. Then from the 1840s it became a gateway to the world. Three new docks - Royal Victoria, Royal Albert and King George V - and the trades that grew around them transformed this area into the industrial heart of the world’s largest port. A busy day in King George V Dock (1965) But this success was not to last. © PLA / Museum of London When the docks closed in 1981 North Woolwich was left isolated and in decline. So a series of projects were established to revive the area, complete with new buildings and transport networks. -

Thames Path Walk Section 4 North Bank Island Gardens to East India
Thames Path Walk Directions: From Island Gardens (once a huge reed bed), admire the Section 4 north bank classical view of Greenwich on the south side. The huge plane trees along the waterfront were planted to hide the industrial buildings inland from the Island Gardens to East India Dock Naval College at Greenwich. There is a small café near the foot tunnel entrance. The opening of the foot tunnel marked the end of the ferry that used to ply its trade from the Ferry House pub, (which still remains to the west). Version 1 : March 2011 From the riverside walk turn left, walk through the gate at the far end of the Start: Island Gardens / Greenwich Foot Gardens, past riverside apartments and under the arcade round the Tunnel (TQ383782) Newcastle drawdock by the Watermans Arms. This area was rebuilt after Station: Island Gardens DLR bombings in WWII. Finish: East India Dock (TQ391807) Station: East India DLR or Canning Town Carry on past apartment blocks until the path is blocked by a remaining industrial warehouse at Storers Quay, now converted to apartments. Walk Distance: 2.5 miles (4.5 km) through the car park behind it to skirt round and rejoin the riverside on its far side. Shortly after passing a private pier on the river the path runs round a Introduction: Beyond the Greenwich Foot Tunnel, the route is no longer shelving beach. designated as a National Trail and is waymarked with the Thames Barge symbol rather than the National Trail acorn. This is because the Thames Path National Trail officially ends on the north bank at Island Gardens on the Follow the riverside path passing an Indian restaurant on the left. -

Lewisham and Greenwich Nhs Trust Trust Strategy, 2021
H LEWISHAM AND GREENWICH NHS TRUST TRUST STRATEGY, 2021-2026 CARING FOR OUR COMMUNITIES Page 159.3 of 159 H Content Page 1. Executive Summary 3 2. Introduction 8 3. Strategic Context 9 • About us • Our Services • Population we serve • Our System Partners 4. Drivers for Change 14 • National Policy, Internal and External drivers • Health needs of our population 5. Our improvement Journey 17 6. Our Strategy 20 1. Quality 2. Patients 3. People 4. Partnerships 5. Money 7. How we get there 26 Appendices 28 Page 159.4 of 159 1. EXECUTIVE SUMMARY H LEWISHAM AND GREENWICH NHS TRUST WAS FORMED IN OCTOBER 2013 The organisation faced early challenges and period of instability. Initial uncertainty about services – the future of some of our key services were unclear – national decision was taken to overturn radical plans for reconfiguration Issues with our workforce – illustrated through high vacancy rates Some progress in service redesign and cross-site working, but this was not consistent Financial instability – we missed our financial targets These factors meant that we faced real challenges progressing our plans for the future. IT WAS CLEAR WE NEEDED TO STABILISE THE TRUST AND SERVICES LGT entered 2018 facing challenges on every front – workforce, quality, performance and finance. This meant that we were compelled to put some of our more ambitious plans, for example for the redevelopment of our sites, on hold while we concentrated on some of the basics. WE HAVE MADE SUBSTANTIAL PROGRESS DURING THE LAST TWO AND A HALF YEARS Three years ago our vacancy rates were among the highest in London (17%), today they are among the lowest (below 9%) • While our overall CQC rating remains Requires Improvement, we have made demonstrable improvement across all areas of our quality dashboard and are now rated good for well led. -

The Transport Committee's Review of the North London Railway March
Transport Committee London’s Forgotten Railway The Transport Committee’s review of the North London Railway March 2006 Transport Committee London’s Forgotten Railway The Transport Committee’s review of the North London Railway March 2006 copyright Greater London Authority March 2006 Published by Greater London Authority City Hall The Queen’s Walk More London London SE1 2AA www.london.gov.uk enquiries 020 7983 4100 minicom 020 7983 4458 ISBN 1 85261 852 3 This publication is printed on recycled paper The Transport Committee Roger Evans - Chairman (Conservative) Geoff Pope - Deputy Chair (Liberal Democrat) John Biggs - Labour Angie Bray - Conservative Elizabeth Howlett - Conservative Peter Hulme Cross - One London Darren Johnson - Green Murad Qureshi - Labour Graham Tope - Liberal Democrat The Transport Committee’s general terms of reference are to examine and report on transport matters of importance to Greater London and the transport strategies, policies and actions of the Mayor, Transport for London, and the other Functional Bodies where appropriate. In particular, the Transport Committee is also required to examine and report to the Assembly from time to time on the Mayor’s Transport Strategy, in particular its implementation and revision. The terms of reference as agreed by the Transport Committee on 20th October 2005 for this scrutiny were: • To survey the current state of the North London Line and the Gospel Oak- Barking line in terms of service frequency, reliability, rolling stock, safety and amenity on stations and station approaches. • To gather and consider the views of Boroughs, business communities, rail passengers, campaign groups and other stakeholders on how they would wish these rail lines to be upgraded and improved.