Wilfred A. Van Der Donk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2020-Commencement-Program.Pdf
One Hundred and Sixty-Second Annual Commencement JUNE 19, 2020 One Hundred and Sixty-Second Annual Commencement 11 A.M. CDT, FRIDAY, JUNE 19, 2020 2982_STUDAFF_CommencementProgram_2020_FRONT.indd 1 6/12/20 12:14 PM UNIVERSITY SEAL AND MOTTO Soon after Northwestern University was founded, its Board of Trustees adopted an official corporate seal. This seal, approved on June 26, 1856, consisted of an open book surrounded by rays of light and circled by the words North western University, Evanston, Illinois. Thirty years later Daniel Bonbright, professor of Latin and a member of Northwestern’s original faculty, redesigned the seal, Whatsoever things are true, retaining the book and light rays and adding two quotations. whatsoever things are honest, On the pages of the open book he placed a Greek quotation from the Gospel of John, chapter 1, verse 14, translating to The Word . whatsoever things are just, full of grace and truth. Circling the book are the first three whatsoever things are pure, words, in Latin, of the University motto: Quaecumque sunt vera whatsoever things are lovely, (What soever things are true). The outer border of the seal carries the name of the University and the date of its founding. This seal, whatsoever things are of good report; which remains Northwestern’s official signature, was approved by if there be any virtue, the Board of Trustees on December 5, 1890. and if there be any praise, The full text of the University motto, adopted on June 17, 1890, is think on these things. from the Epistle of Paul the Apostle to the Philippians, chapter 4, verse 8 (King James Version). -

The Nakanishi Symposium on Natural Products & Bioorganic Chemistry
The Nakanishi Symposium on Natural Products & Bioorganic Chemistry March 19, 2021 Sponsored by The Chemical Society of Japan & The American Chemical Society ー1ー Yoshito Kishi Professor Emeritus, Harvard University ■EDUCATION Bachelor of Science, Nagoya University 1961 Doctor of Philosophy (Chemistry), Nagoya University (Professors Yoshimasa Hirata and Toshio Goto) 1966 Postdoctoral Research Fellow (Chemistry), Harvard University (Professor R. B. Woodward) 1966-1968 ■ACADEMIC APPOINTMENT Instructor of Chemistry, Nagoya University 1966-1970 Associate Professor of Agricultural Chemistry, Nagoya University 1970-1974 Visiting Professor of Chemistry, Harvard University 1972-1973 Professor of Chemistry, Harvard University 1974-1982 Morris Loeb Professor of Chemistry, Harvard University 1982-2002 Morris Loeb Professor of Chemistry, Emeritus, Harvard University 2002- ー2ー ■RESEARCH TOPICS (chronological order) 1. Chemical studies of bioluminescence: The luminescent substance named luciferin is an unstable compound, making its structural determination extremely difficult. Dr. Kishi, however, determined the structures of luciferins, such as those in Cypridina, Genji fireflies, krill, dinoflagellates, and the luminous shellfish of Latia (1960s–1980s). 2. Total synthesis of complex natural products: The pufferfish toxin tetrodotoxin, the structure of which was determined in 1964, is still known as one of the most difficult natural products to synthesize due to its highly functionalized structural complexity. Dr. Kishi achieved the world’s first total synthesis of this compound in 1972. Later, he achieved total synthesis of the paralytic shellfish toxin saxitoxin, the anticancer drug mitomycin C, the fungus toxin sporidesmin, and the β-lactam antibiotics (1970s–1980s). 3. Development of acyclic stereocontrol in total synthesis of natural products: Until the early 1970s, total synthesis of polyether antibiotics was almost impossible due to the presence of numerous asymmetric centers. -

Canada – USSR Hockey Exchanges. Between Positive and Negative Sports Diplomacy *
Historia i Polityka No. 18 (25)/2016, pp. 19–32 ISSN 1899-5160, e-ISSN 2391-7652 www.hip.umk.pl DOI: http://dx.doi.org/10.12775/HiP.2016.029 Michał Marcin Kobierecki University of Lodz, Poland Canada – USSR Hockey Exchanges. Between Positive and Negative Sports Diplomacy * Kontakty hokejowe Kanada – ZSRR. Pomiędzy pozytywną a negatywną dyplomacją sportową • A bst ra kt • • A bst ract • Celem artykułu jest zbadanie dyplomacji hoke- The aim of the article is to investigate the issue jowej pomiędzy Kanadą a Związkiem Radziec- of hockey diplomacy between Canada and the kim, do jakiej doszło w latach siedemdziesią- Soviet Union, which was held in 1970s. It en- tych XX wieku. Obejmowała ona organizację compassed a series of exhibition matches in ice szeregu meczów w hokeju na lodzie, których hockey, which were directly aimed to improve bezpośrednim celem było nawiązanie bliższych relations between the two states belonging to relacji pomiędzy dwoma należącymi do prze- different Cold War alliances. ciwstawnych bloków geopolitycznych krajami. In the article an attempt to verify a num- W artykule podjęta została próba weryfika- ber of hypotheses was made. According to the cji szeregu hipotez badawczych. Główna zakła- main one, the hockey exchanges were in fact da, iż dyplomacja hokejowa była w rzeczywisto- a fusion of positive and negative sports diplo- ści połączeniem elementów pozytywnej i nega- macy. The second hypothesis states that hockey tywnej dyplomacji sportowej. Zgodnie z kolej- diplomacy was at the same time an effect and ną, kontakty hokejowe były zarazem przejawem a tool of Canadian and Soviet desire to better i narzędziem zbliżenia pomiędzy krajami, nato- their bilateral relations, while according to the miast według ostatniej hipotezy wybór hokeja last one, selection of ice hockey was adequate na lodzie jako narzędzia dyplomatycznego był concerning the diplomatic objective of political adekwatny. -

The Member Magazine of the American Society for Biochemistry and Molecular Biology CONTENTS
Vol. 13 / No. 5 / May 2014 THE MEMBER MAGAZINE OF THE AMERICAN SOCIETY FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY CONTENTS NEWS FEATURES PERSPECTIVES 2 14 18 PRESIDENT’S MESSAGE PROPAGATING POSSIBILITIES OPEN LETTER Good reads and the power of data Researcher tinkers with tree genetics On hindsight and gratitude 5 19 NEWS FROM THE HILL PROFESSIONAL DEVELOPMENT Making scientic research 11 19 Tips for Ruth L. Kirschstein training a priority for Congress grant applicants 21 e skills you need for 6 a career in science policy MEMBER UPDATE 22 Give credit where it is due ACS honors nine ASBMB members 24 7 EDUCATION JOURNAL NEWS 24 Reimagining the undergraduate 18 science course 12 27 ‘Creativity is in all – not a possession ASBMB NEWS of only a certain few’ 2014 annual meeting travel award winners 28 OUTREACH Yale Science Diplomats 28 31 LIPID NEWS Desperately seeking Sputnik for fundamental science 32 OPEN CHANNELS Reader comments 14 12 In our cover story, we learn about one research team’s eort to manipulate common trees to produce high-value commodities. MAY 2014 ASBMB TODAY 1 PRESIDENT’S MESSAGE calibration curves are much worse. tomy for breast cancer by surgeon perpetually changing landscape of is is particularly true for the local William Halsted and the implications breast cancer was beginning to tire THE MEMBER MAGAZINE OF THE AMERICAN SOCIETY television forecasts: ey substantially of studies of its eectiveness. Moving him out. Trials, tables, and charts FOR BIOCHEMISTRY AND MOLECULAR BIOLOGY Good reads and overpredict the probability of rain. past surgical treatments that focused had never been his forte; he was a is tendency gets at a key point. -
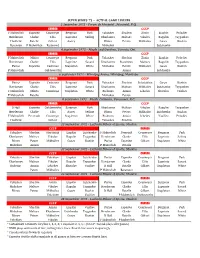
1972 "Summit Series"
SUPER SERIES '72 -- ACTUAL GAME LINEUPS 2 September 1972 - Forum de Montréal , Montréal, P.Q. CANADA CCCP F Mahovlich Esposito Cournoyer Bergman Park Yakushev Shadrin Zimin Liapkin Poladiev Henderson Clarke Ellis Lapointe Seiling Kharlamov Maltsev Vikulov Ragulin Tsygankov Hadfield Ratelle Gilbert Awrey Blinov Petrov Mikhailov Gusev Kuzkin Berenson P Mahovlich Redmond Mishakov Lutchenko 4 September 1972 - Maple Leaf Gardens, Toronto, Ont. CANADA CCCP F Mahovlich Mikita Cournoyer Bergman Park Yakushev Shadrin Zimin Liapkin Poladiev Henderson Clarke Ellis Lapointe Savard Kharlamov Starshinov Maltsev Ragulin Tsygankov Parise Esposito Cashman Stapleton White Mishakov Petrov Mikhailov Gusev Kuzkin P Mahovlich Goldsworthy Anisin Lutchenko 6 September 1972 - Winnipeg Arena, Winnipeg, Manitoba CANADA CCCP Parise Esposito Cashman Bergman Park Yakushev Shadrin Solodukhin Gusev Kuzkin Henderson Clarke Ellis Lapointe Savard Kharlamov Maltsev Mikhailov Lutchenko Tsygankov F Mahovlich Mikita Cournoyer Stapleton White Bodonov Anisin Lebedev Shatalov Vasiliev P Mahovlich Ratelle Mishakov Petrov 8 September 1972 - Pacific Coliseum, Vancouver, B.C. CANADA CCCP D Hull Esposito Goldsworthy Bergman Park Kharlamov Maltsev Vikulov Ragulin Tsygankov Henderson Clarke Ellis Awrey Seiling Blinov Petrov Mikhailov Lutchenko Kuzkin F Mahovlich Perreault Cournoyer Stapleton White Bodonov Anisin Lebedev Vasiliev Poladiev Hadfield Gilbert Yakushev Shadrin 22 September 1972 - Luzhniki Palace of Sports, Moskva CCCP CANADA Yakushev Shadrin Martiniuk Liapkin Lutchenko F -

Robert A. Alberty 1921–2014
Robert A. Alberty 1921–2014 A Biographical Memoir by Gordon G. Hammes and Carl Frieden ©2014 National Academy of Sciences. Any opinions expressed in this memoir are those of the authors and do not necessarily reflect the views of the National Academy of Sciences. ROBERT ARNOLD ALBERTY June 21, 1921–January 18, 2014 Elected to the NAS, 1965 Robert A. Alberty maintained an enthusiasm for science throughout his entire life. His presence at meetings was easily detectable, as he was blessed with an unmistak- able and booming voice that conveyed his latest scien- tific interests and above all his continual commitment to the science enterprise. Alberty directed this passion to thermodynamics and kinetics in particular, especially as applied to biological systems, and his research in these areas established a rich legacy for modern biophysical chemistry. He was a “triple-threat” scientist, excelling not only in research but also in teaching and university administration. Alberty (Bob to all who knew him) was born in Winfield, By Gordon G. Hammes Kansas, but when he was five years old his family moved and Carl Frieden to Lincoln, Nebraska. Even as a boy, he displayed a strong interest in science, exemplified by a basement chemistry laboratory and photographic dark room that he built in the family’s home. When he entered the University of Nebraska in 1939, Alberty had been planning a career as a chemical engineer, but then he discovered that this would require extensive coursework in drafting and surveying. Because he had already learned surveying from his grandfather, he saw no need to take college courses in the subject. -

Newsletter University of Wisconsin-Madison
NEWSLETTER UNIVERSITY OF WISCONSIN-MADISON For friends of the Department of Biochemistry at the University of Wisconsin–Madison Table of Contents From the Chair .............................................................. 3 Gene Regulation .....................................................20-21 Exciting Discoveries ...................................................4-6 In Memoriam ..............................................................21 Biochemistry Phase II ...................................................7 Remembering Ross Inman ..........................................22 Kamaluddin Ahmad Graduate Scholarship ...................8 RNA Maxi Group ........................................................23 Student Faculty Liaison Committee ..............................9 Department Alumnus: Dustin Maly ............................ 24 After Retirement - Bill Reznikoff .................................10 Department Alumnus: Jenifer (Bork) Miskowski ....... 25 New Faculty Profi le .....................................................11 Biochemistry Graduate Degrees .............................26-29 Celebrating Har Gobind Khorana ..........................12-13 Staff Departures ..........................................................30 Our Department in India .......................................14-15 From the Labs ........................................................31-53 Our Department in Uganda ........................................16 Contact Information ...................................................54 iGEM -

15/5/40 Liberal Arts and Sciences Chemistry Irwin C. Gunsalus Papers, 1877-1993 BIOGRAPHICAL NOTE Irwin C
15/5/40 Liberal Arts and Sciences Chemistry Irwin C. Gunsalus Papers, 1877-1993 BIOGRAPHICAL NOTE Irwin C. Gunsalus 1912 Born in South Dakota, son of Irwin Clyde and Anna Shea Gunsalus 1935 B.S. in Bacteriology, Cornell University 1937 M.S. in Bacteriology, Cornell University 1940 Ph.D. in Bacteriology, Cornell University 1940-44 Assistant Professor of Bacteriology, Cornell University 1944-46 Associate Professor of Bacteriology, Cornell University 1946-47 Professor of Bacteriology, Cornell University 1947-50 Professor of Bacteriology, Indiana University 1949 John Simon Guggenheim Fellow 1950-55 Professor of Microbiology, University of Illinois 1955-82 Professor of Biochemistry, University of Illinois 1955-66 Head of Division of Biochemistry, University of Illinois 1959 John Simon Guggenheim Fellow 1959-60 Research sabbatical, Institut Edmund de Rothchild, Paris 1962 Patent granted for lipoic acid 1965- Member of National Academy of Sciences 1968 John Simon Guggenheim Fellow 1972-76 Member Levis Faculty Center Board of Directors 1977-78 Research sabbatical, Institut Edmund de Rothchild, Paris 1973-75 President of Levis Faculty Center Board of Directors 1978-81 Chairman of National Academy of Sciences, Section of Biochemistry 1982- Professor of Biochemistry, Emeritus, University of Illinois 1984 Honorary Doctorate, Indiana University 15/5/40 2 Box Contents List Box Contents Box Number Biographical and Personal Biographical Materials, 1967-1995 1 Personal Finances, 1961-65 1-2 Publications, Studies and Reports Journals and Reports, 1955-68 -

Past, Present & Future Collide in Canada
08_2061_IIHF_IceTimes_Vol12-3 2.7.2008 14:30 Uhr Seite 1 July 2008 Volume 12 Number 3 Published by International Ice Hockey Federation Editor-in-Chief Horst Lichtner Editor Szymon Szemberg Design Jenny Wiedeke Past, present & future collide in Canada GENERATIONS MEET: During the 2008 World Championship in Canada, the IIHF honored its colorful past by recognizing the 1980 USA Miracle on Ice as the top story of the Century (bottom left Rene Fasel, Walter L. Bush Jr. 1980 player Mark Johnson, Art Berglund and IOC President Jacques Rogge mark the milestone). Also recognized was the Centennial All-Star Team (bottom right - see page 4). The IIHF also ushered in a new generation of stars as a young Russian team, led by Alexander Ovehkin, captured the 2008 gold medal. Also on the minds of those in Canada, the formation of the new CHL, where European Clubs, including top attendended Bern in Switzerland, will play. IIHF pays homage to hockey legends and welcomes next generation After recognizing and honouring our past in Quebec City, behind the first organized game of at the Victoria Skating Rink in Montreal in 1875. it's time to look forward. The General Congress in Montreal gave the new council and myself a new four- II It's time now to roll up our sleeves and try to make international hockey even bet- year mandate to lead the IIHF until 2012. I take on this ter in the four years to come. We are facing many challenges. As most people know, task with great modesty and pride. the IIHF-NHL transfer agreement has expired, which means that the relationship bet- ween the IIHF sphere and the NHL is not regulated. -

Splendid Condition and Enormous 'Grit'": the Ps Orting "Other" and Canadian Identity
Dialogues: Undergraduate Research in Philosophy, History, and Politics Volume 3 Article 2 2020 "Splendid Condition and Enormous 'Grit'": The pS orting "Other" and Canadian Identity Cassidy L. Jean Thompson Rivers University Follow this and additional works at: https://digitalcommons.library.tru.ca/phpdialogues Part of the History Commons Recommended Citation Jean, Cassidy L. (2020) ""Splendid Condition and Enormous 'Grit'": The pS orting "Other" and Canadian Identity," Dialogues: Undergraduate Research in Philosophy, History, and Politics: Vol. 3 , Article 2. Available at: https://digitalcommons.library.tru.ca/phpdialogues/vol3/iss1/2 This Article is brought to you for free and open access by Digital Commons @ TRU Library. It has been accepted for inclusion in Dialogues: Undergraduate Research in Philosophy, History, and Politics by an authorized editor of Digital Commons @ TRU Library. For more information, please contact [email protected]. "Splendid Condition and Enormous 'Grit'": The pS orting "Other" and Canadian Identity Abstract Canadian identity is mutable, changing in response to outside influences. This phenomenon is especially apparent in sport. This paper focuses on the formation and maintenance of Canadian identity in sport. By connecting the 1867 Paris rowing crew, the 1972 Summit Series, and the 2019 NBA champion Toronto Raptors, this paper seeks to investigate how Canadian identity has been shaped through sport. Using newspaper articles, online editorials, and academic sources, this paper shows how integral the sporting “other” is to the Canadian identity. Keywords Sport, Identity, Canadian, Summit Series, Toronto Raptors, History, Paris Crew, Media Studies This article is available in Dialogues: Undergraduate Research in Philosophy, History, and Politics: https://digitalcommons.library.tru.ca/phpdialogues/vol3/iss1/2 Jean: The Sporting "Other" and Canadian Identity “Splendid Condition and Enormous ‘Grit’:” The Sporting “Other” and Canadian Identity Cassidy L. -

Chemical Compositions the Magazine of the Department of Chemistry and Biochemistry the University of Texas at Austin OH O O O O O O O O H N H O N 1
Fall l 2008 CC Chemical Compositions The Magazine of The Department of Chemistry and Biochemistry The University of Texas at Austin O OH O O O O O O O H N H O N 1. nBuLi O OTBS O N ∆ Br Br MeO2C N 2. R"X R R" O OH Pd Enolate N 2 CO2H S S N Me R' R" N N H Me Arylation N R R' R' Lewis Acid Mediated Me Me R N S S N Me O Coupling H O O OH 1 3 4 OH NH2 OAc O OH O O OH O H H H O Allylic TMS i. 4Å MS, CH3CN MeO2C (-)-gliotoxin MeO2C MeO2C ii. TFA, -45 °C to rt O O O Alkylation O + O OMe HO N N N OMe N Me Me Me H N NH iii. NaCN aq., CH2Cl2 emestrin 5 6 7 N 1. nBuLi N ∆ 2 N 2. R"X OMe CN 1-pot R R" 89% (88:12) NCbz O R R' R' Cbz R R" e.g. N O BnO N R' NH2 O R1 H HO H Cl R2 1 2 R O + R N N O Ts OH H Br M R Ar R N N Ar O Me OH Cl Me OH Me H Br N Me N N H NH Me p-toluenesulfonic acid H Me O Me HO o O H 0 C O Me (-)-epimyrtine (±)-tashiromine CN Me N 30 min Ts (-)-Caryophyllene Oxide 30% Br (HO) OP O ∆G ∆H T∆S 2 (HO)2OP O Compounda -1 Ka (M ) (kcal/mol) (kcal/mol) (kcal/mol) 1 [1.0 ± 0.1] x 106 -8.2 ± 0.1 -7.0 ± 0.1 1.2 ± 0.03 2 [4.4 ± 0.4] x 105 -7.7 ± 0.1 -5.3 ± 0.1 2.4 ± 0.06 O Acyclic O 7 O Control O 3 [8.5 ± 0.3] x 105 -8.1 ± 0.1 -6.2 ± 0.02 1.9 ± 0.02 N N Acyclic 2 H H 5 Control HN O HN (3) ([5.6 ± 0.2] x 10 ) (-7.9 ± 0.1) (-4.9 ± 0.02) (3.0 ± 0.02) HN R R R R Macro- 4 [9.9 ± 2.9] x 106 -9.5 ± 0.2 -4.8 ± 0.1 4.7 ± 0.2 HN O cyclization O HN O N N N N NH HN (4) ([6.5 ± 0.9] x 106) (-9.3 ± 0.1) (-6.4 ± 0.04) (2.9 ± 0.1) Macro- cyclization H O N N O a Results in parentheses refer to corresponding acyclic controls. -

League Equivalencies – ����������������
League Equivalencies – Gabriel Desjardins League Equivalencies ’a goal in one league worth in another? NHL teams draft and sign players from numerous major junior leagues, US and Canadian colleges, European Elite Leagues and various North American minor leagues. In evaluating players, it is critical to know how a ’performance will translate to the NHL. Other things being equal, the quality of one league relative to another is reflected in how many points a player would score in each league. Research has shown that while NHL pla’playing time and point scoring peaks between ages 23 and 26, their point-per-game (PPG) production reaches 90% of its peak value much earlier, and holds its value beyond age 30. It is rare that a player plays significant time in two leagues in the same year, but players often play in one league in one year and another the next. With a large number of such players, we can then estimate the quality of each league using the ratio of the ’PPG in each year. What we are most interested in is what one point in a given league is equivalent to in the NHL – in other words, the league equivalency. There is enough data to use the above method to estimate the league equivalence for the best minor leagues (AHL/IHL), most major professional leagues (European Elite Leagues) and the WHA. Minor Leagues With the demise of the International Hockey League, the AHL is now the top minor professional league in North America. But how good is it? Obviously, there are no serious games between NHL teams and their AHL affiliates.