P2Y1R Inhibitors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

P2Y Purinergic Receptors Regulate the Growth of Human Melanomas
Cancer Letters 224 (2005) 81–91 www.elsevier.com/locate/canlet P2Y purinergic receptors regulate the growth of human melanomas Nicholas Whitea,b, Mina Rytena, Elizabeth Claytonc, Peter Butlerb, Geoffrey Burnstocka,* aAutonomic Neuroscience Institute, Royal Free and University College Medical School, Rowland Hill Street, London NW3 2PF, UK bDepartment of Plastic and Reconstructive Surgery, Royal Free Hospital, Pond Street, London NW3 2QG, UK cRAFT Institute of Plastic Surgery, Mount Vernon Hospital, Northwood, Middlesex HA6 2RN, UK Received 3 October 2004; received in revised form 6 November 2004; accepted 9 November 2004 Abstract Adenosine 50-triphosphate is known to function as a potent extracellular messenger producing its effects via a distinct family of cell surface receptors. Different receptor subtypes have been shown to modulate different cellular functions such as proliferation, differentiation and apoptosis. We investigated the functional expression and proliferative action of metabotropic P2Y receptors in human melanoma tissue and cells. Expression of functional P2Y1, P2Y2 and P2Y6 receptor subtypes was established by reverse transcriptase polymerase chain reaction, immunohistochemistry and intracellular calcium measurements using a Fluorometric Imaging Plate Reader. Incubation of A375 melanoma cells with the P2Y1 receptor-selective agonist 2- methylthioadenosine-5-diphosphate caused a decrease in cell number which was dose-dependent, whereas incubation with the P2Y2 receptor agonist uridine triphosphate caused a dose-dependent increase in cell number. The action of extracellular nucleotides on P2Y receptors was shown to mediate the growth of melanomas and the P2Y1 receptor is a putative target for melanoma therapy. q 2004 Elsevier Ireland Ltd. All rights reserved. Keywords: Cancer; Melanoma; P2Y receptor; ATP 1. -

P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases
International Journal of Molecular Sciences Review P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases Derek Strassheim 1, Alexander Verin 2, Robert Batori 2 , Hala Nijmeh 3, Nana Burns 1, Anita Kovacs-Kasa 2, Nagavedi S. Umapathy 4, Janavi Kotamarthi 5, Yash S. Gokhale 5, Vijaya Karoor 1, Kurt R. Stenmark 1,3 and Evgenia Gerasimovskaya 1,3,* 1 The Department of Medicine Cardiovascular and Pulmonary Research Laboratory, University of Colorado Denver, Aurora, CO 80045, USA; [email protected] (D.S.); [email protected] (N.B.); [email protected] (V.K.); [email protected] (K.R.S.) 2 Vascular Biology Center, Augusta University, Augusta, GA 30912, USA; [email protected] (A.V.); [email protected] (R.B.); [email protected] (A.K.-K.) 3 The Department of Pediatrics, Division of Critical Care Medicine, University of Colorado Denver, Aurora, CO 80045, USA; [email protected] 4 Center for Blood Disorders, Augusta University, Augusta, GA 30912, USA; [email protected] 5 The Department of BioMedical Engineering, University of Wisconsin, Madison, WI 53706, USA; [email protected] (J.K.); [email protected] (Y.S.G.) * Correspondence: [email protected]; Tel.: +1-303-724-5614 Received: 25 August 2020; Accepted: 15 September 2020; Published: 18 September 2020 Abstract: Purinergic G-protein-coupled receptors are ancient and the most abundant group of G-protein-coupled receptors (GPCRs). The wide distribution of purinergic receptors in the cardiovascular system, together with the expression of multiple receptor subtypes in endothelial cells (ECs) and other vascular cells demonstrates the physiological importance of the purinergic signaling system in the regulation of the cardiovascular system. -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. -

G Protein‐Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: G protein-coupled receptors. British Journal of Pharmacology (2019) 176, S21–S141 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors Stephen PH Alexander1 , Arthur Christopoulos2 , Anthony P Davenport3 , Eamonn Kelly4, Alistair Mathie5 , John A Peters6 , Emma L Veale5 ,JaneFArmstrong7 , Elena Faccenda7 ,SimonDHarding7 ,AdamJPawson7 , Joanna L Sharman7 , Christopher Southan7 , Jamie A Davies7 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK 2Monash Institute of Pharmaceutical Sciences and Department of Pharmacology, Monash University, Parkville, Victoria 3052, Australia 3Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK 4School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, BS8 1TD, UK 5Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway, Anson Building, Central Avenue, Chatham Maritime, Chatham, Kent, ME4 4TB, UK 6Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK 7Centre for Discovery Brain Sciences, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2019/20 is the fourth in this series of biennial publications. The Concise Guide provides concise overviews of the key properties of nearly 1800 human drug targets with an emphasis on selective pharmacology (where available), plus links to the open access knowledgebase source of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. Although the Concise Guide represents approximately 400 pages, the material presented is substantially reduced compared to information and links presented on the website. -

Purinergic Receptors Brian F
Chapter 21 Purinergic receptors Brian F. King and Geoffrey Burnstock 21.1 Introduction The term purinergic receptor (or purinoceptor) was first introduced to describe classes of membrane receptors that, when activated by either neurally released ATP (P2 purinoceptor) or its breakdown product adenosine (P1 purinoceptor), mediated relaxation of gut smooth muscle (Burnstock 1972, 1978). P2 purinoceptors were further divided into five broad phenotypes (P2X, P2Y, P2Z, P2U, and P2T) according to pharmacological profile and tissue distribution (Burnstock and Kennedy 1985; Gordon 1986; O’Connor et al. 1991; Dubyak 1991). Thereafter, they were reorganized into families of metabotropic ATP receptors (P2Y, P2U, and P2T) and ionotropic ATP receptors (P2X and P2Z) (Dubyak and El-Moatassim 1993), later redefined as extended P2Y and P2X families (Abbracchio and Burnstock 1994). In the early 1990s, cDNAs were isolated for three heptahelical proteins—called P2Y1, P2Y2, and P2Y3—with structural similarities to the rhodopsin GPCR template. At first, these three GPCRs were believed to correspond to the P2Y, P2U, and P2T receptors. However, the com- plexity of the P2Y receptor family was underestimated. At least 15, possibly 16, heptahelical proteins have been associated with the P2Y receptor family (King et al. 2001, see Table 21.1). Multiple expression of P2Y receptors is considered the norm in all tissues (Ralevic and Burnstock 1998) and mixtures of P2 purinoceptors have been reported in central neurones (Chessell et al. 1997) and glia (King et al. 1996). The situation is compounded by P2Y protein dimerization to generate receptor assemblies with subtly distinct pharmacological proper- ties from their constituent components (Filippov et al. -

P2X and P2Y Receptors
Tocris Scientific Review Series Tocri-lu-2945 P2X and P2Y Receptors Kenneth A. Jacobson Subtypes and Structures of P2 Receptor Molecular Recognition Section, Laboratory of Bioorganic Families Chemistry, National Institute of Diabetes and Digestive and The P2 receptors for extracellular nucleotides are widely Kidney Diseases, National Institutes of Health, Bethesda, distributed in the body and participate in regulation of nearly Maryland 20892, USA. E-mail: [email protected] every physiological process.1,2 Of particular interest are nucleotide Kenneth Jacobson serves as Chief of the Laboratory of Bioorganic receptors in the immune, inflammatory, cardiovascular, muscular, Chemistry and the Molecular Recognition Section at the National and central and peripheral nervous systems. The ubiquitous Institute of Diabetes and Digestive and Kidney Diseases, National signaling properties of extracellular nucleotides acting at two Institutes of Health in Bethesda, Maryland, USA. Dr. Jacobson is distinct families of P2 receptors – fast P2X ion channels and P2Y a medicinal chemist with interests in the structure and receptors (G-protein-coupled receptors) – are now well pharmacology of G-protein-coupled receptors, in particular recognized. These extracellular nucleotides are produced in receptors for adenosine and for purine and pyrimidine response to tissue stress and cell damage and in the processes nucleotides. of neurotransmitter release and channel formation. Their concentrations can vary dramatically depending on circumstances. Thus, the state of activation of these receptors can be highly dependent on the stress conditions or disease states affecting a given organ. The P2 receptors respond to various extracellular mono- and dinucleotides (Table 1). The P2X receptors are more structurally restrictive than P2Y receptors in agonist selectivity. -

Lack of Adipocyte Purinergic P2Y6 Receptor Greatly Improves Whole Body Glucose Homeostasis
Lack of adipocyte purinergic P2Y6 receptor greatly improves whole body glucose homeostasis Shanu Jaina, Sai P. Pydib, Kiran S. Totia, Bernard Robayec, Marco Idzkod,e, Oksana Gavrilovaf, Jürgen Wessb, and Kenneth A. Jacobsona,1 aMolecular Recognition Section, Laboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD 20892; bMolecular Signaling Section, Laboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD 20892; cInstitute of Interdisciplinary Research (IRIBHM), Université Libre de Bruxelles, 6041 Gosselies, Belgium; dUniversitätsklinik für Innere Medizin II, Klinische Abteilung für Pulmologie, Medizinische Universität, 1090 Vienna, Austria; eDepartment of Pneumology, University Hospital Freiburg, 79106 Freiburg, Germany; and fMouse Metabolism Core, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD 20892 Edited by Robert J. Lefkowitz, Howard Hughes Medical Institute, Durham, NC, and approved October 7, 2020 (received for review April 7, 2020) Uridine diphosphate (UDP)-activated purinergic receptor P2Y6 activation by uridine diphosphate (UDP) increases glucose uptake (P2Y6R) plays a crucial role in controlling energy balance through in adipocyte 3T3-L1 and skeletal muscle C2C12 cell lines (15). ’ central mechanisms. However, P2Y6R s roles in peripheral tissues P2Y6R also plays a pivotal role in inflammatory responses by regulating energy and glucose homeostasis remain unexplored. stimulating immune cell migration and inducing the secretion of Here, we report the surprising finding that adipocyte-specific de- inflammatory cytokines such as MCP1 and IL6 (16, 17). Stresses letion of P2Y6R protects mice from diet-induced obesity, improving such as environmental inflammation, chronic heart failure, and glucose tolerance and insulin sensitivity with reduced systemic in- dystrophic cardiomyopathy increase P2Y6R expression (18, 19). -

Adenosine, an Endogenous Distress Signal, Modulates Tissue Damage and Repair
Cell Death and Differentiation (2007) 14, 1315–1323 & 2007 Nature Publishing Group All rights reserved 1350-9047/07 $30.00 www.nature.com/cdd Review Adenosine, an endogenous distress signal, modulates tissue damage and repair BB Fredholm*,1 Adenosine is formed inside cells or on their surface, mostly by breakdown of adenine nucleotides. The formation of adenosine increases in different conditions of stress and distress. Adenosine acts on four G-protein coupled receptors: two of them, A1 and A3, are primarily coupled to Gi family G proteins; and two of them, A2A and A2B, are mostly coupled to Gs like G proteins. These receptors are antagonized by xanthines including caffeine. Via these receptors it affects many cells and organs, usually having a cytoprotective function. Joel Linden1 recently grouped these protective effects into four general modes of action: increased oxygen supply/demand ratio, preconditioning, anti-inflammatory effects and stimulation of angiogenesis. This review will briefly summarize what is known and what is not in this regard. It is argued that drugs targeting adenosine receptors might be useful adjuncts in many therapeutic approaches. Cell Death and Differentiation (2007) 14, 1315–1323; doi:10.1038/sj.cdd.4402132; published online 30 March 2007 Adenosine Formation and Adenosine Levels out of the cells by means of efficient equilibrative transporters. There are inhibitors for these transporters, including the drug Adenosine is always present both within and outside cells, dipyridamole. These inhibitors will increase extracellular since it is at a crossroads between different metabolic adenosine if it derives from extracellular breakdown of pathways. Levels of adenosine in cells and tissue fluids is adenine nucleotides, but decrease it if adenosine is formed in the nanomolar range under physiological conditions intracellularly. -

The P2Y2 Receptor Sensitizes Mouse Bladder Sensory Neurons and Facilitates Purinergic Currents
The Journal of Neuroscience, February 10, 2010 • 30(6):2365–2372 • 2365 Neurobiology of Disease The P2Y2 Receptor Sensitizes Mouse Bladder Sensory Neurons and Facilitates Purinergic Currents Xiaowei Chen,1 Derek C. Molliver,2 and G. F. Gebhart1 Center for Pain Research, Departments of 1Anesthesiology and 2Medicine, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania 15213 Sensitization of bladder afferents is an underlying contributor to the development and maintenance of painful bladder syndrome/ interstitial cystitis. Extracellular purines and pyrimidines (e.g., ATP and UTP), released during bladder distension or from damaged cells after tissue insult, are thought to play an important role in bladder physiological and pathological states by actions at ionotropic P2X and metabotropic P2Y receptors. In the present study, we examined the ability of P2Y receptors to sensitize and modulate P2X-mediated responses in mouse bladder sensory neurons. UTP (a P2Y2 and P2Y4 agonist) increased excitability of bladder neurons by depolarizing resting membrane potential, increasing action potential firing, and facilitating responses to suprathreshold current injection as well as to P2X agonist application. These effects of UTP on bladder neuron excitability were blocked by the P2Y2 receptor antagonist suramin. UTP also facilitated bladder neuron homomeric P2X2 sustained currents and homomeric P2X3 fast currents. The facilitatory effect of UTP on P2X2 sustained currents was mediated by a G-protein-coupled P2Y2 receptor/PKC pathway, whereas the effect of UTP on P2X3 fast currents was G-protein independent. We also examined P2X and P2Y receptor expression in bladder neurons. P2Y2 and P2Y4 transcripts ϳ ϳ were detected in 50 and 20% of bladder neurons, respectively. -

Molecular and Functional Characterization of P2Y12 Expression in Tumor-Associated Macrophages
Aus der Klinik für Dermatologie, Venerologie und Allergologie Universitätsklinikum Mannheim der Medizinischen Fakultät Mannheim der Ruprecht-Karls-Universität Heidelberg (Direktor: Prof. Dr. med. S. Goerdt) Molecular and Functional Characterization of P2Y12 Expression in Tumor-associated Macrophages INAUGURALDISSERTATION zur Erlangung der Doktorwürde der Naturwissenschaftlich-Mathematischen Gesamtfakultät der Ruprecht-Karls-Universität Heidelberg Vorgelegt von Loreen Kloss DISSERTATION submitted to the Combined Faculty of Natural Sciences and Mathematics of the Ruperto Carola University Heidelberg, Germany for the degree of Doctor of Natural Sciences Presented by Loreen Kloss, M.Sc. Born in: Herrenberg, Germany Oral examination: June 17th, 2019 Molecular and Functional Characterization of P2Y12 Expression in Tumor-associated Macrophages Referees: Prof. Dr. rer. nat. Viktor Umansky PD Dr. med. Astrid Schmieder Table of Contents Table of Contents Abstract ...................................................................................................................................... V Zusammenfassung ................................................................................................................... VII 1. Introduction ....................................................................................................................... 1 1.1 Macrophages ............................................................................................................... 1 1.1.1 Origin and maintenance of macrophages ........................................................... -
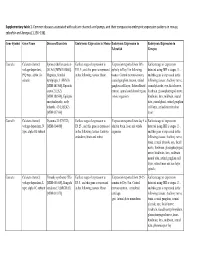
Supplementary Tables 1 Final.Xlsx
Supplementary table 1. Common diseases associated with calcium channels and pumps, and their comparative embryonic expression patterns in mouse, zebrafish and Xenopus [1,136–138]. Gene Symbol Gene Name Diseases/Disorders Embryonic Expression in Mouse Embryonic Expression in Embryonic Expression in Zebrafish Xenopus Cacna1a Calcium channel, Spinocerebellar ataxia 6 Earliest stage of expression is Expression reported from 50% Earliest stage of expression voltage-dependent, (SCA6) [MIM:183086], E11.5 , and this gene is expressed epiboly to Day 5 in following detected using ISH is stages 13 , P/Q type, alpha 1A Migraine, familial in the following tissues: Brain tissues: Central nervous system, and this gene is expressed in the subunit hemiplegic, 1 (FHM1) cranial ganglion, neuron, retinal following tissues: Auditory nerve, [MIM:141500], Episodic ganglion cell layer, Rohon-Beard cranial placode, eye, facial nerve, ataxia 2 (EA2) neuron, spinal cord dorsal region, forebrain, glossopharyngeal nerve, [MIM:108500], Epileptic whole organism hindbrain, lens, midbrain, neural encephalopathy, early tube, pineal gland, retinal ganglion infantile, 42 (EIEE42) cell layer, retinal inner nuclear [MIM:617106] layer Cacna1b Calcium channel, Dystonia 23 (DYT23) Earliest stage of expression is Expression reported from day 5 to Earliest stage of expression voltage-dependent, N [MIM:614860] E8.25 , and this gene is expressed adult in brain, liver and whole detected using ISH is stages 13 , type, alpha 1B subunit in the following tissues: Embryo organism and this -

Control of Macrophage Inflammation by P2Y Purinergic
cells Review Control of Macrophage Inflammation by P2Y Purinergic Receptors Dominik Klaver and Martin Thurnher * Immunotherapy Unit, Department of Urology, Medical University of Innsbruck, 6020 Innsbruck, Austria; [email protected] * Correspondence: [email protected]; Tel.: +43-512-504-24867 Abstract: Macrophages comprise a phenotypically and functionally diverse group of hematopoietic cells. Versatile macrophage subsets engage to ensure maintenance of tissue integrity. To perform tissue stress surveillance, macrophages express many different stress-sensing receptors, including purinergic P2X and P2Y receptors that respond to extracellular nucleotides and their sugar derivatives. Activation of G protein-coupled P2Y receptors can be both pro- and anti-inflammatory. Current examples include the observation that P2Y14 receptor promotes STAT1-mediated inflammation in pro-inflammatory M1 macrophages as well as the demonstration that P2Y11 receptor suppresses the secretion of tumor necrosis factor (TNF)-α and concomitantly promotes the release of soluble TNF receptors from anti-inflammatory M2 macrophages. Here, we review macrophage regulation by P2Y purinergic receptors, both in physiological and disease-associated inflammation. Therapeutic targeting of anti-inflammatory P2Y receptor signaling is desirable to attenuate excessive inflammation in infectious diseases such as COVID-19. Conversely, anti-inflammatory P2Y receptor signaling must be suppressed during cancer therapy to preserve its efficacy. Keywords: P2Y receptor; G protein; P2Y11; P2Y14; ATP; UDP-glucose; macrophage; inflammation Citation: Klaver, D.; Thurnher, M. Control of Macrophage Inflammation by P2Y Purinergic Receptors. Cells 1. Introduction 2021, 10, 1098. https://doi.org/ 10.3390/cells10051098 Elie Metchnikoff (1845–1916) not only first described macrophages but also recognized that a fundamental characteristic of these cells is to strive for balance [1].