An Evaluation of Growth and Body Composition of Droughtmaster
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Markers for Polled, African Horn and Scurs Genes in Tropical Beef Cattle
final reportp Project code: B.AHW.0144 Prepared by: Dr John Henshall CSIRO Livestock Industries Date published: November 2011 ISBN: 9781741916683 PUBLISHED BY Meat & Livestock Australia Limited Locked Bag 991 NORTH SYDNEY NSW 2059 Genetic markers for polled, African Horn and Scurs genes in tropical beef cattle Meat & Livestock Australia acknowledges the matching funds provided by the Australian Government to support the research and development detailed in this publication. This publication is published by Meat & Livestock Australia Limited ABN 39 081 678 364 (MLA). Care is taken to ensure the accuracy of the information contained in this publication. However MLA cannot accept responsibility for the accuracy or completeness of the information or opinions contained in the publication. You should make your own enquiries before making decisions concerning your interests. Reproduction in whole or in part of this publication is prohibited without prior written consent of MLA. Genetic markers for polled, African Horn and Scurs genes in tropical beef cattle Abstract The objective of this project was to develop gene marker tests for the Polled, Scurs and African Horn genes. A microsatellite marker was found that is very closely associated with the polled locus in Brahman, Santa Gertrudis, Hereford, Droughtmaster and Limousin. In these breeds animals homozygous for the allele associated with polled almost never have horns or scurs, so no additional test for the scurs or African horn gene is required. In Tropical Composite cattle, those that are homozygous for the allele associated with polled are most likely to be polled, but also may be horned or scurred. This may be due to incomplete association, or due to another region of the chromosome such as the hypothesised African Horn gene. -

Southeast Asia Acknowledgements the Subregional Factsheet Was Prepared by Marieke Reuver
Subregional Report on Animal Genetic Resources: Southeast Asia Acknowledgements The Subregional Factsheet was prepared by Marieke Reuver. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by the Food and Agriculture Organization of the United Nations in preference to others of a similar nature that are not mentioned. The views expressed in this publication are those of the author(s) and do not necessarily reflect the views of the Food and Agriculture Organization of the United Nations. All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to: Chief Electronic Publishing Policy and Support Branch Communication Division FAO Viale delle Terme di Caracalla, 00153 Rome, Italy or by e-mail to: [email protected] © FAO 2007 Citation: FAO. 2007. Subregional report on animal genetic resources: Southeast Asia. Annex to The State of the World’s Animal Genetic Resources for Food and Agriculture. -

Productivity of Pure Imported Droughtmaster and F1 Droughtmaster and Lai Sind Cattle in South- Eastern Region of Vietnam
PRODUCTIVITY OF PURE IMPORTED DROUGHTMASTER AND F1 DROUGHTMASTER AND LAI SIND CATTLE IN SOUTH- EASTERN REGION OF VIETNAM Pham Van Quyen INTRODUCTION In recent years, market demand on beef has inceased following the economic growth and consumers’ preference. However, domestic beef products are low in quality and quantity compared to market demand. Each year, the country has to import a large amount of high quanlity beef. Droughtmaster was developed in Australia. It is a mixed tropical cattle breed with 50% Shorthorn and 50% Brahman. It has high tolerance to hot and humid conditions, resist to tick and good reproduction. They have been mainly kept at Queensland State, the tropics of Northern Australia. Droughtmaster were imported to Vietnam from 2002 - 2003. Performance sturdies pure Droughtmaster and their cross-bred calves in Vietnam had been conducted. MATERIALS AND METHODS Content 1: Studying appearance characteristics, growth and reproductivity of Droughtmaster: Experiment was conducted at Ruminant Research and Training Center (RRTC), Lai Hung, Ben Cat, Binh Duong province from March 2003 to August 2007 on 30 Droughtmaster female heifers, 14 – 18 months old, imported from Australia. Content 2: Studying appearance characteristics, growth of F1 Droughtmaster x Lai Sind: Experiment was conducted at RRTC from Octerber, 2002 to August, 2007. 155 Lai Sind female cattle, 18 – 36 months old, 220 kg of body weight were used to inseminate Droughtmaster, Brahman, Charolais semen to produce F1. Content 3: Studying efficiency of fattening on Droughtmaster male and F1 Droughtmaster x Lai Sind male: Experiment was conducted at RRTC from August to December 2005, using 15 beef male cattle, 15 – 18 months old, 220 - 350 kg of body weight from 5 breeding groups: Droughtmaster, F1 Droughtmaster, F1 Brahman, F1 Charolais and Lai Sind, 3head/group. -

Breeds of Beef and Multi-Purpose Cattle
BREEDS OF BEEF AND MULTI-PURPOSE CATTLE ACKNOWLEDGEMENTS The inspiration for writing this book goes back to my undergraduate student days at Iowa State University when I enrolled in the course, “Breeds of Livestock,” taught by the late Dr. Roy Kottman, who was then the Associate Dean of Agriculture for Undergraduate Instruction. I was also inspired by my livestock judging team coach, Professor James Kiser, who took us to many great livestock breeders’ farms for practice judging workouts. I also wish to acknowledge the late Dr. Ronald H. Nelson, former Chairman of the Department of Animal Science at Michigan State University. Dr. Nelson offered me an Instructorship position in 1957 to pursue an advanced degree as well as teach a number of undergraduate courses, including “Breeds of Livestock.” I enjoyed my work so much that I never left, and remained at Michigan State for my entire 47-year career in Animal Science. During this career, I had an opportunity to judge shows involving a significant number of the breeds of cattle reviewed in this book. I wish to acknowledge the various associations who invited me to judge their shows and become acquainted with their breeders. Furthermore, I want to express thanks to my spouse, Dr. Leah Cox Ritchie, for her patience while working on this book, and to Ms. Nancy Perkins for her expertise in typing the original manuscript. I also want to acknowledge the late Dr. Hilton Briggs, the author of the textbook, “Modern Breeds of Livestock.” I admired him greatly and was honored to become his close friend in the later years of his life. -

B.NBP.0759 Final Report
final report Project code: B.NBP.0759 Prepared by: David Johnston1, Kirsty Moore1, Jim Cook1 and Tim Grant2 1Animal Genetics and Breeding Unit, UNE, Armidale 2Queensland Department of Agriculture and Fisheries, Toowoomba Date published: 26 March 2019 PUBLISHED BY Meat and Livestock Australia Limited Locked Bag 1961 NORTH SYDNEY NSW 2059 Enabling genetic improvement of reproduction in tropical beef cattle Meat & Livestock Australia acknowledges the matching funds provided by the Australian Government to support the research and development detailed in this publication. This publication is published by Meat & Livestock Australia Limited ABN 39 081 678 364 (MLA). Care is taken to ensure the accuracy of the information contained in this publication. However MLA cannot accept responsibility for the accuracy or completeness of the information or opinions contained in the publication. You should make your own enquiries before making decisions concerning your interests. Reproduction in whole or in part of this publication is prohibited without prior written consent of MLA. B.NBP.0759 – Enabling genetic improvement of reproduction in tropical breed Abstract Reproduction rate is a key profit driver in many northern Australian production systems. Genetics is a tool that can increase performance, however difficulties in recording and later expression of the traits associated with female reproduction have meant little or no genetic progress. Genomic selection has emerged as powerful new tool in livestock breeding but has had little application in beef due to low numbers genotyped. This Project used intensive recording and genotyping to build genomic reference populations for three tropical beef breeds. This is enabling the application of genomic selection as a result of significant increases in the accuracy of the female reproduction EBV days to calving (DC) in all three tropical breeds, and increased numbers of young bulls with DC EBVs. -

Sire Directory 2020
Australia 2020 - 2021 AUSTRALIAN BEEF SIRE DIRECTORY FOREWORD 2020-212019-20 INDEX INDEX FOREWORD Page Page PRICE LIST ................................... 24, 25 PRICE LIST ................................26, 27 SYNCHRONIZATION BENEFITSSYNCRONIZATION....... 48, IBC BENEFITS ..... 52, IBC ABS HCR PROGRAMABS ......................... HCR PROGRAM2, 3 ..................30, 31 ABS SEXCEL .......................................ABS SEXCEL ...................................27 14 ANGUS ......................................ABS SOCIAL 1-23,MEDIA 26 ........................ 39 Bill Cornell Fletch Kelly Kim Sultana Beef Product Manager Beef Key Account Manager Beef Key Account ManagerRED ANGUS .................................. 28, 29 Bill Cornell Beef Key Account Manager ABS BEEF SYMBOLS ....................... 25 Victoria, SA, Tasmania Northern Region Beef Product Manager Southern Region BELGIAN BLUE ................................... 30 Australia and New Zealand ANGUS ........................1-13, 15-25, 28 ABS Australia continues to2019-20 remain in the No. 1INDEX industry position for beef FOREWORD BELTED GALLOWAY .............................ANGUS TOP 10 ...............................30 29 semen sales in Australia. Page The NHIA (National Herd Improvement Association) semen sire survey, to BRANGUS ..........................................RED ANGUS ...............................30 32, 33 which all member A.I. companiesPRICE LIST submit ................................ their annual returns,26, 27showed ABS Australia Beef has 42% of the domestic -

By Harlan Ritchie BREEDS of BEEF and MULTI-PURPOSE CATTLE
2009 By Harlan Ritchie BREEDS OF BEEF AND MULTI-PURPOSE CATTLE ACKNOWLEDGEMENTS The inspiration for writing this book goes back to my undergraduate student days at Iowa State University when I enrolled in the course, “Breeds of Livestock,” taught by the late Dr. Roy Kottman, who was then the Associate Dean of Agriculture for Undergraduate Instruction. I was also inspired by my livestock judging team coach, Professor James Kiser, who took us to many great livestock breeders’ farms for practice judging workouts. I also wish to acknowledge the late Dr. Ronald H. Nelson, former Chairman of the Department of Animal Science at Michigan State University. Dr. Nelson offered me an Instructorship position in 1957 to pursue an advanced degree as well as teach a number of undergraduate courses, including “Breeds of Livestock.” I enjoyed my work so much that I never left, and remained at Michigan State for my entire 47-year career in Animal Science. During this career, I had an opportunity to judge shows involving a significant number of the breeds of cattle reviewed in this book. I wish to acknowledge the various associations who invited me to judge their shows and become acquainted with their breeders. Furthermore, I want to express thanks to my spouse, Dr. Leah Cox Ritchie, for her patience while working on this book, and to Ms. Nancy Perkins for her expertise in typing the original manuscript. I also want to acknowledge the late Dr. Hilton Briggs, the author of the textbook, “Modern Breeds of Livestock.” I admired him greatly and was honored to become his close friend in the later years of his life. -
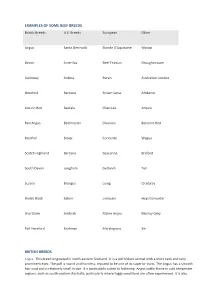
EXAMPLES of SOME BEEF BREEDS British Breeds U.S
EXAMPLES OF SOME BEEF BREEDS British Breeds U.S. Breeds European Other Angus Santa Gertrudis Blonde D'Aquitaine Watusi Devon Amerifax Beef Friesian Droughtmaster Galloway Ankina Boran Australian Lowline Hereford Barzona Brown Swiss Afrikaner Lincoln Red Beefalo Charolais Ankole Red Angus Beefmaster Chianina Belmont Red Red Poll Braler Corriente Wagyu Scotch Highland Barzona Gasconne Braford South Devon Longhorn Gelbvieh Tuli Sussex Brangus Luing Charbray Welsh Black Salorn Limousin Hays Converter Shorthorn Simbrah Maine Anjou Murray Grey Poll Hereford Brahman Marchigiana Siri BRITISH BREEDS Angus: This breed originated in north-eastern Scotland. It is a pitch black animal with a short neck and very prominent eyes. The poll is round and hornless, reputed to be one of its superior traits. The Angus has a smooth hair coat and is relatively small in size. It is particularly suited to fattening. Angus cattle thrive in cold temperate regions, such as south-eastern Australia, particularly where foggy conditions are often experienced. It is also very useful for cross-breeding in those areas. Desirable traits include mothering and milking ability, early maturity, easy calving, easy mustering, high fertility and an extremely good carcass quality. Hereford: The Hereford evolved in the south western part of England on the border of Wales and is one of most well known breeds in the world. It has a reddish brown body and a characteristic white face. They are medium to large in size and are horned (preferably growing downward and inward). Desirable traits include hardiness, grazing ability, rugged adaptability, reproductive efficiency, good temperament, heavy bones and thick flesh. Tendency towards some health problems (e.g. -

Diversity 2014, 6 S1 Supplementary Information Table S1. Landraces
Diversity 2014, 6 S1 Supplementary Information Table S1. Landraces, varieties, pre-breeds and breeds absorbed into current breeds (Felius, 1995; Porter 2002). Names in local language, if not English, are in italics. Bold indicate current breeds resulting from amalgamations; small printing indicate varieties of a landrace, pre-breed or former breed. Years underlined indicate the establishment of a herd book with for a few breeds also the ending; HB, herd book established but year unknown; BS, breed society with year of establishment if known; BP, protective breed program with year of establishment if known. Populations listed in the first two columns have been absorbed in the current breed (in bold), populations in the fourth column have been absorbed after the current breed was established. Names on the same line indicate continuation of a population under a different name. “×breed X” indicates incrossing of breed X; “breed Y × breed X” indicates upgrading or incrossing of breed X by breed Y; +breed Y indicates influence of breed Y. Landrace, Pre-breed, Current breed Absorbed variety Remark variety former breed Subgroup 1A Westland Polled Lyngdal South and Westland (1947) Westland Red Polled 1968 into NRF, 1980s restarted Blacksided Trondheim Northland Blacksided Trondheim and Northland (1943) close to Fjällras Roros crossbred cattle Swedish Mountain (Fjällras) (1892) Herjeadals Rorbottenland Estonian land cattle Estonian Native (1914) West Finn and Jersey influence Petsjora now Kholmogory variety Komi Subgroup 1B Telemark (1926) Ayrshire -

Global Benchmarking Results for Beef Producers
How are global and Australian beef producers performing? Global agri benchmark network results 2016 Written by Karl Behrendt (Charles Sturt University) and Peter Weeks (Weeks Consulting Services) Commissioned by Meat & Livestock Australia January 2017 MLA Market Information Report – How are global and Australian beef producers performing? Contents Highlights – Beef cattle ......................................................1 Introduction .........................................................................1 What is agri benchmark?....................................................1 Global price and cost trends ............................................. 4 Food and meat prices ................................................................. 4 Global cattle price trends ........................................................... 4 Cattle price forecasts ......................................................... 5 Changes in projections ...............................................................5 OECD-FAO demand and price projections ..............................5 USDA price forecasts ..................................................................6 Grain prices ......................................................................... 7 Global meat supply ............................................................ 8 World beef supply .............................................................. 9 Beef consumption ............................................................ 10 Beef trade ......................................................................... -
The Queensland Beef Supply Chain
The Queensland Beef Supply Chain The Queensland Beef Cattle Supply Chain | 1 Ernst & Young Australia Operations Pty Limited Tel: +61 7 3011 3333 111 Eagle Street Fax: +61 7 3011 3100 Brisbane QLD 4000 Australia ey.com/au GPO Box 7878 Brisbane QLD 4001 A message from the Queensland Department of Agriculture and Fisheries ‘The department is committed to providing accessible content for the widest possible audience. We are actively working to improve our offering. If this document does not meet your needs please contact us on 13 25 23 for assistance.’ Release Notice EY was engaged on the instructions of the Queensland Department of Agriculture and Fisheries (“DAF”) to develop the ‘Investment Outlook for the Queensland Beef Supply Chain’ document series, in accordance with the agreement (the “Agreement”) dated 27 March 2018. The results of EY’s work are set out across six reports. This report (the “Report”) is one of the six reports. EY has prepared the Reports for the benefit of DAF and has considered only the interests of DAF. We formed these opinions through desktop research, EY subject matter resources and analysis. EY has not be engaged to act, and has not acted, as an advisor to any other party. Accordingly, EY makes no representations as to the appropriateness, accuracy or completeness of the Reports for any other party’s purposes. The Report will be used for the purpose of providing current information on the sector (the “Purpose”). This Report was prepared on the specific instructions of DAF solely for the Purpose and should not be used or relied upon for any other purpose or by anyone else for any purpose. -

Comparison of Genetic Merit for Weight and Meat Traits Between the Polled and Horned Cattle in Multiple Beef Breeds
animals Article Comparison of Genetic Merit for Weight and Meat Traits between the Polled and Horned Cattle in Multiple Beef Breeds Imtiaz A. S. Randhawa 1,* , Michael R. McGowan 1 , Laercio R. Porto-Neto 2, Ben J. Hayes 3 and Russell E. Lyons 1,4 1 School of Veterinary Science, University of Queensland, Gatton, QLD 4343, Australia; [email protected] (M.R.M.); [email protected] (R.E.L.) 2 CSIRO Agriculture and Food, St Lucia, QLD 4067, Australia; [email protected] 3 Centre for Animal Science, Queensland Alliance for Agriculture and Food Innovation, University of Queensland, St Lucia, QLD 4072, Australia; [email protected] 4 Agri-Genetics Consulting, Brisbane, QLD 4074, Australia * Correspondence: [email protected] Simple Summary: Beef production has expanded worldwide through cattle adaptation to diverse environmental and husbandry conditions. The beef industry faces societal challenges from animal welfare perspectives, including dehorning and disbudding, which are common farm practices to limit animal and handler injuries by the horned cattle. Most cattle breeds were originally horned, and reluctance to poll breeding existed because of perceived negative correlations between polledness and production. In Australia, population trends indicate a recent rise to above 50% of poll types in six breeds (Charolais, Hereford, Limousin, Simmental, Shorthorn, and Droughtmaster), and two breeds with lower but increasing rates of polledness (Brahman and Santa Gertrudis). Overall, recently estimated breeding values of 12 investigated production traits have not shown consistently negative Citation: Randhawa, I.A.S.; trends within and across breeds. Thus, polledness should not be considered as detrimental.