Autophagy Is Activated for Cell Survival After Endoplasmic Reticulum Stress
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Autophagy Controls Centrosome Number
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 9), pp: 14277-14278 Editorial: Autophagy and Cell Death Autophagy controls centrosome number Shinya Honda and Shigeomi Shimizu It is believed that the number of centrosomes in a that in normal cells, and (4) silencing Cep63 decreased cell is tightly regulated by the degradation of centrosomal the number of centrosomes in autophagy-deficient cells. proteins via the ubiquitin–proteasome protein degradation From these findings, we concluded that Cep63 dots are the system. However, we recently demonstrated that direct substrates of autophagy and multiple centrosomes autophagy also participates in the regulation of centrosome are generated from the excess Cep63 dots in autophagy- number. deficient cells. The centrosome is the major component of the Consistent with these findings, in autophagy- microtubule organizing center of mammalian cells, deficient cells, most Cep63 dots directly bound to p62, and plays an essential role in chromosome segregation an adaptor or cargo receptor for autophagic degradation. during cell division. Centrosome number is tightly These p62-associated Cep63 dots were not become mature regulated during the cell cycle: G1 cells have one mature centrosomes, whereas some Cep63 dots become mature centrosome containing a pair of centrioles. Centriole centrosomes by their interaction with Cep152, instead of replication occurs during the S phase, and each centriole p62. Cep152 is the first protein to interact with Cep63 in duplicates to produce two daughter centrioles, to generate the initial step of centriole duplication (Figure 1). two centrosomes in the G2–M phase. If cells have more Our data indicated that Cep63 dots are continuously than three centrosomes during metaphase, this causes generated and rapidly degraded by autophagy. -

Autophagy Regulation by RNA Decay
On the edge of degradation: Autophagy regulation by RNA decay Elizabeth Delorme-Axford1 and Daniel J. Klionsky1# From the 1Life Sciences Institute, University of Michigan, Ann Arbor, MI 48109, USA # Correspondence to: Daniel J. Klionsky; University of Michigan; Life Sciences Institute; Rm. 6036; 210 Washtenaw Ave.; Ann Arbor, Michigan 48109-2216 USA; Email: [email protected] Keywords: Dcp2, DDX6, Dhh1, mRNA decay, RNA degradation, vacuole, Xrn1/XRN1, yeast Abbreviations: ATG, autophagy-related; CPA, cleavage and polyadenylation; Cvt, cytoplasm- to-vacuole targeting; GABARAP, GABA type A receptor-associated protein; MAP1LC3/ LC3, microtubule associated protein 1 light chain 3; miRNA, microRNA; mRNA, messenger RNA; MTOR, mechanistic target of rapamycin kinase; NMD, nonsense-mediated decay; qRT-PCR, quantitative real-time PCR; SG, stress granule; siRNA, small interfering RNA; TOR, target of rapamycin; TORC1, TOR complex 1; Ubl, ubiquitin-like; UTR, untranslated region This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/wrna.1522 1 This article is protected by copyright. All rights reserved. Running title: Autophagy regulation by RNA decay Abstract Cells must dynamically adapt to altered environmental conditions, particularly during times of stress, to ensure their ability to function effectively and survive. The macroautophagy/autophagy pathway is highly conserved across eukaryotic cells and promotes cell survival during stressful conditions. In general, basal autophagy occurs at a low level to sustain cellular homeostasis and metabolism. -

Autophagy: from Basic Science to Clinical Application
nature publishing group REVIEW See COMMENTARY page XX Autophagy: from basic science to clinical application J Va n L i m b e r g e n 1 , 2 , 3 , C S t e v e n s 4 , E R N i m m o 1 , D C W i l s o n 2 , 3 a n d J S a t s a n g i 1 Autophagy is a cellular pathway involved in protein and organelle degradation, which is likely to represent an innate adaptation to starvation. In times of nutrient deficiency, the cell can self-digest and recycle some nonessential components through nonselective autophagy, thus sustaining minimal growth requirements until a food source becomes available. Over recent years, autophagy has been implicated in an increasing number of clinical scenarios, notably infectious diseases, cancer, neurodegenerative diseases, and autoimmunity. The recent identification of the importance of autophagy genes in the genetic susceptibility to Crohn ’ s disease suggests that a selective autophagic response may play a crucial role in the pathogenesis of common complex immune-mediated diseases. In this review, we discuss the autophagic mechanisms, their molecular regulation, and summarize their clinical relevance. This progress has led to great interest in the therapeutic potential of manipulation of both selective and nonselective autophagy in established disease. INTRODUCTION The ability to adapt to environmental change is essential for sur- Autophagy encompasses several distinct processes involving vival. This is true for the organism as a whole and for individual the delivery of portions of the cytoplasm to the lysosome for cells alike. -

The Endomembrane System and Proteins
Chapter 4 | Cell Structure 121 Endosymbiosis We have mentioned that both mitochondria and chloroplasts contain DNA and ribosomes. Have you wondered why? Strong evidence points to endosymbiosis as the explanation. Symbiosis is a relationship in which organisms from two separate species depend on each other for their survival. Endosymbiosis (endo- = “within”) is a mutually beneficial relationship in which one organism lives inside the other. Endosymbiotic relationships abound in nature. We have already mentioned that microbes that produce vitamin K live inside the human gut. This relationship is beneficial for us because we are unable to synthesize vitamin K. It is also beneficial for the microbes because they are protected from other organisms and from drying out, and they receive abundant food from the environment of the large intestine. Scientists have long noticed that bacteria, mitochondria, and chloroplasts are similar in size. We also know that bacteria have DNA and ribosomes, just like mitochondria and chloroplasts. Scientists believe that host cells and bacteria formed an endosymbiotic relationship when the host cells ingested both aerobic and autotrophic bacteria (cyanobacteria) but did not destroy them. Through many millions of years of evolution, these ingested bacteria became more specialized in their functions, with the aerobic bacteria becoming mitochondria and the autotrophic bacteria becoming chloroplasts. The Central Vacuole Previously, we mentioned vacuoles as essential components of plant cells. If you look at Figure 4.8b, you will see that plant cells each have a large central vacuole that occupies most of the cell's area. The central vacuole plays a key role in regulating the cell’s concentration of water in changing environmental conditions. -

PEX5 Regulates Autophagy Via the Mtorc1-TFEB Axis During Starvation
Eun et al. Experimental & Molecular Medicine (2018) 50:4 DOI 10.1038/s12276-017-0007-8 Experimental & Molecular Medicine ARTICLE Open Access PEX5 regulates autophagy via the mTORC1-TFEB axis during starvation So Young Eun1,JoonNoLee2,In-KooNam2, Zhi-qiang Liu1,Hong-SeobSo 1, Seong-Kyu Choe1 and RaeKil Park2 Abstract Defects in the PEX5 gene impair the import of peroxisomal matrix proteins, leading to nonfunctional peroxisomes and other associated pathological defects such as Zellweger syndrome. Although PEX5 regulates autophagy process in a stress condition, the mechanisms controlling autophagy by PEX5 under nutrient deprivation are largely unknown. Herein, we show a novel function of PEX5 in the regulation of autophagy via Transcription Factor EB (TFEB). Under serum-starved conditions, when PEX5 is depleted, the mammalian target of rapamycin (mTORC1) inhibitor TSC2 is downregulated, which results in increased phosphorylation of the mTORC1 substrates, including 70S6K, S6K, and 4E- BP-1. mTORC1 activation further suppresses the nuclear localization of TFEB, as indicated by decreased mRNA levels of TFEB, LIPA, and LAMP1. Interestingly, peroxisomal mRNA and protein levels are also reduced by TFEB inactivation, indicating that TFEB might control peroxisome biogenesis at a transcriptional level. Conversely, pharmacological inhibition of mTOR resulting from PEX5 depletion during nutrient starvation activates TFEB by promoting nuclear localization of the protein. In addition, mTORC1 inhibition recovers the damaged-peroxisome biogenesis. These data suggest that PEX5 may be a critical regulator of lysosomal gene expression and autophagy through the mTOR-TFEB- autophagy axis under nutrient deprivation. 1234567890():,; 1234567890():,; Introduction Mitochondrial antiviral-signaling protein (MAVS) func- Peroxisome is an essential cellular organelle for per- tions as an antiviral signaling platform to induce the forming various metabolic activities, including oxidation interferon-independent signaling pathways4. -

Autophagy of an Amyloid-Like Translational Repressor Regulates Meiotic Exit
Short Article Autophagy of an Amyloid-like Translational Repressor Regulates Meiotic Exit Highlights Authors d Autophagy is required for meiotic exit Fei Wang, Rudian Zhang, Wenzhi Feng, ..., Jiajia Li, d Autophagy prevents additional rounds of chromosome Vladimir Denic, Soni Lacefield segregation after meiosis II Correspondence d Autophagy degrades the amyloid-like translational repressor Rim4 in meiosis II [email protected] (F.W.), [email protected] (V.D.), [email protected] (S.L.) d Inhibition of autophagy leads to aberrant persistence of meiotic regulators In Brief Wang et al. report that autophagy promotes meiotic termination by degrading Rim4, an amyloid-like translational repressor whose timed clearance in meiosis II regulates protein production from its mRNA targets. In the absence of autophagy, cells fail to exit meiosis and undergo additional rounds of chromosome segregation after meiosis II. Wang et al., 2020, Developmental Cell 52, 141–151 January 27, 2020 ª 2019 Elsevier Inc. https://doi.org/10.1016/j.devcel.2019.12.017 Developmental Cell Short Article Autophagy of an Amyloid-like Translational Repressor Regulates Meiotic Exit Fei Wang,1,* Rudian Zhang,1 Wenzhi Feng,1 Dai Tsuchiya,2,4 Olivia Ballew,2 Jiajia Li,1 Vladimir Denic,3,* and Soni Lacefield2,5,* 1Department of Internal Medicine, Center for Autophagy Research, UT Southwestern Medical Center, Dallas, TX, USA 2Department of Biology, Indiana University, Bloomington, IN, USA 3Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA, USA 4Present address: Stowers Institute for Medical Research, Kansas City, MO, USA 5Lead Contact *Correspondence: [email protected] (F.W.), [email protected] (V.D.), [email protected] (S.L.) https://doi.org/10.1016/j.devcel.2019.12.017 SUMMARY events during different stages of sexual reproduction and early stages of development (Yin et al., 2016). -

Reflux of Endoplasmic Reticulum Proteins to the Cytosol Yields Inactivation of Tumor Suppressors
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.13.038935; this version posted April 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Reflux of Endoplasmic Reticulum proteins to the cytosol yields inactivation of tumor suppressors Daria Sicari1,2, Raphael Pineau1,2, Pierre-Jean Le Reste1,2,3, Luc Negroni4,5,6,7, Sophie Chat8, Aiman Mohtar9, Daniel Thomas8, Reynald Gillet8, M. Ted Hupp9,10, Eric Chevet1,2* and Aeid Igbaria1,2,11* 1Inserm U1242, University of Rennes, Rennes, France. 2Centre de lutte contre le cancer Eugène Marquis, Rennes, France. 3Neurosurgery Dept, University Hospital of Rennes, 35000 Rennes, France. 4Institut de Génétique et de Biologie Moléculaire et Cellulaire, 67404 Illkirch, France. 5Centre National de la Recherche Scientifique, UMR7104, 67404 Illkirch, France. 6Institut National de la Santé et de la Recherche Médicale, U1258, 67404 Illkirch, France. 7Université de Strasbourg, 67404 Illkirch, France. 8Univ. Rennes, CNRS, Institut de Génétique et Développement de Rennes (IGDR) UMR6290, 35000 Rennes, France. 9Edinburgh Cancer Research Centre at the Institute of Genetics and Molecular Medicine, Edinburgh University, Edinburgh, UK. 10International Centre for Cancer Vaccine Science, Gdansk, Poland. 11Department of Life Sciences, Ben-Gurion University of the Negev, Beer Sheva 8410501, Israel. *Correspondence: [email protected], or [email protected] ABSTRACT: In the past decades many studies reported Endoplasmic Reticulum (ER) resident proteins to localize to the cytosol but the mechanisms by which this occurs and whether these proteins exert cytosolic functions remain unknown. We found that select ER luminal proteins accumulate in the cytosol of glioblastoma cells isolated from mouse and human tumors. -

Autophagy in the Endocrine Glands
A WECKMAN and others Autophagy in the endocrine 52:2 R151–R163 Review glands Autophagy in the endocrine glands Andrea Weckman, Antonio Di Ieva, Fabio Rotondo1, Luis V Syro2, Leon D Ortiz3, Kalman Kovacs1 and Michael D Cusimano Division of Neurosurgery, Department of Surgery, St Michael’s Hospital, University of Toronto, Toronto, Ontario, Canada Correspondence 1Division of Pathology, Department of Laboratory Medicine, St Michael’s Hospital, University of Toronto, Toronto, should be addressed Ontario, Canada to A Di Ieva 2Department of Neurosurgery, Hospital Pablo Tobon Uribe and Clinica Medellin, Medellin, Colombia Email 3Division of Neurooncology, Instituto de Cancerologia, Clinic Las Americas, Medellin, Colombia [email protected] Abstract Autophagy is an important cellular process involving the degradation of intracellular Key Words components. Its regulation is complex and while there are many methods available, there is " autophagy currently no single effective way of detecting and monitoring autophagy. It has several " endocrine glands cellular functions that are conserved throughout the body, as well as a variety of different " crinophagy physiological roles depending on the context of its occurrence in the body. Autophagy is also " endocrine diseases involved in the pathology of a wide range of diseases. Within the endocrine system, autophagy has both its traditional conserved functions and specific functions. In the endocrine glands, autophagy plays a critical role in controlling intracellular hormone levels. In peptide-secreting cells of glands such as the pituitary gland, crinophagy, a specific form of autophagy, targets the secretory granules to control the levels of stored hormone. In steroid-secreting cells of glands such as the testes and adrenal gland, autophagy targets the steroid-producing organelles. -

ER-Phagy at a Glance Paolo Grumati1,*, Ivan Dikic1,2,‡ and Alexandra Stolz2,*
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs217364. doi:10.1242/jcs.217364 CELL SCIENCE AT A GLANCE ER-phagy at a glance Paolo Grumati1,*, Ivan Dikic1,2,‡ and Alexandra Stolz2,* ABSTRACT function in response to ER stress signals. This task sharing reflects Selective autophagy represents the major quality control mechanism the complexity of the ER in terms of biological functions and that ensures proper turnover of exhausted or harmful organelles, morphology. In this Cell Science at a Glance article and the among them the endoplasmic reticulum (ER), which is fragmented accompanying poster, we summarize the most recent findings and delivered to the lysosome for degradation via a specific type of about ER-phagy in yeast and in mammalian cells. autophagy called ER-phagy. The recent discovery of ER-resident KEY WORDS: Autophagy, CCPG1, FAM134B, RTN3, SEC62, proteins that bind to mammalian Atg8 proteins has revealed that the Endoplasmic reticulum selective elimination of ER involves different receptors that are specific for different ER subdomains or ER stresses. FAM134B (also known as RETREG1) and RTN3 are reticulon-type proteins that are Introduction able to remodel the ER network and ensure the basal membrane The endoplasmic reticulum (ER) is the largest membrane-bound turnover. SEC62 and CCPG1 are transmembrane ER receptors that organelle in eukaryotic cells. Its complex morphology, which involves sheets, tubules and matrices (Chen et al., 2013; Friedman and Voeltz, 2011; Nixon-Abell et al., 2016), mirrors its diverse roles 1Institute of Biochemistry II, Goethe University Frankfurt - Medical Faculty, in a variety of physiological processes including autophagy University Hospital, 60590 Frankfurt am Main, Germany. -
Endomembrane System
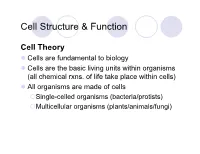
Cell Structure & Function Cell Theory Cells are fundamental to biology Cells are the basic living units within organisms (all chemical rxns. of life take place within cells) All organisms are made of cells Single-celled organisms (bacteria/protists) Multicellular organisms (plants/animals/fungi) Cell Structure & Function Basic Aspects of Cell Structure & Function Plasma membrane Lipid bilayer Proteins DNA-containing region Cytoplasm Eukaryotic v. Prokaryotic cells Prokaryotic v. Eukaryotic Cells Two major classes of cells Prokaryotic cells (pro-, “before”) Cell lacks a “true” nucleus DNA is coiled in a nucleoid region Cells lack nuclear membrane Prokaryotic v. Eukaryotic Cells [attachment structure] [DNA location] [organelles that synthesize proteins] [enclosing the cytoplasm] [rigid structure outside the p.m. ] [jelly-like outer coating] [locomotion organelle] Prokaryotic v. Eukaryotic Cells Eukaryotic cells (eu-, “true”) Nucleus contains most of the cells nuclear material, DNA usually the largest organelle Bordered by a membranous envelope Prokaryotic v. Eukaryotic Cells Plant v. Animal Cells Both contain Plasma membrane (functions as a selective barrier) Nucleus (gene-containing organelle) Cytoplasm (region between nucleus and p.m.) Consists of organelles in a fluid (cytosol) Prokaryotic v. Eukaryotic Cells Plant v. Animal Cells Organelles Bordered by internal membranes Compartmentalizes the functions of a cell Maintains organelle’s unique environment Most organelles are found in both plant and animal cells Plant v. Animal Cells -

ER-Phagy and Its Role in ER Homeostasis in Plants
plants Review ER-Phagy and Its Role in ER Homeostasis in Plants Yan Bao 1,2,* and Diane C. Bassham 1,* 1 Department of Genetics, Development and Cell Biology, Iowa State University, Ames, IA 50011, USA 2 Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI 48824, USA * Correspondence: [email protected] (Y.B.); [email protected] (D.C.B.) Received: 19 November 2020; Accepted: 11 December 2020; Published: 14 December 2020 Abstract: The endoplasmic reticulum (ER) is the largest continuous membrane-bound cellular organelle and plays a central role in the biosynthesis of lipids and proteins and their distribution to other organelles. Autophagy is a conserved process that is required for recycling unwanted cellular components. Recent studies have implicated the ER as a membrane source for the formation of autophagosomes, vesicles that transport material to the vacuole during autophagy. When unfolded proteins accumulate in the ER and/or the ER lipid bilayer is disrupted, a condition known as ER stress results. During ER stress, ER membranes can also be engulfed through autophagy in a process termed ER-phagy. An interplay between ER stress responses and autophagy thus maintains the functions of the ER to allow cellular survival. In this review, we discuss recent progress in understanding ER-phagy in plants, including identification of regulatory factors and selective autophagy receptors. We also identify key unanswered questions in plant ER-phagy for future study. Keywords: autophagy; endoplasmic reticulum; ER stress; ER-phagy; unfolded protein response 1. Introduction Plants live in a world of ever-changing conditions; for survival, they need to adapt to the challenges of their surroundings to balance growth and stress responses [1,2]. -

Autophagy Stimulus-Dependent Role of the Small Gtpase Ras2 in Peroxisome Degradation
biomolecules Communication Autophagy Stimulus-Dependent Role of the Small GTPase Ras2 in Peroxisome Degradation Fahd Boutouja 1,2 and Harald W. Platta 1,* 1 Biochemie Intrazellulärer Transportprozesse, Ruhr-Universität Bochum, 44801 Bochum, Germany; [email protected] 2 Institute of Pathobiochemistry, Johannes Gutenberg-University Mainz, 55099 Mainz, Germany * Correspondence: [email protected]; Tel.: +49-234-322-4968 Received: 17 October 2020; Accepted: 12 November 2020; Published: 14 November 2020 Abstract: The changing accessibility of nutrient resources induces the reprogramming of cellular metabolism in order to adapt the cell to the altered growth conditions. The nutrient-depending signaling depends on the kinases mTOR (mechanistic target of rapamycin), which is mainly activated by nitrogen-resources, and PKA (protein kinase A), which is mainly activated by glucose, as well as both of their associated factors. These systems promote protein synthesis and cell proliferation, while they inhibit degradation of cellular content by unselective bulk autophagy. Much less is known about their role in selective autophagy pathways, which have a more regulated cellular function. Especially, we were interested to analyse the central Ras2-module of the PKA-pathway in the context of peroxisome degradation. Yeast Ras2 is homologous to the mammalian Ras proteins, whose mutant forms are responsible for 33% of human cancers. In the present study, we were able to demonstrate a context-dependent role of Ras2 activity depending on the type of mTOR-inhibition and glucose-sensing situation. When mTOR was inhibited directly via the macrolide rapamycin, peroxisome degradation was still partially suppressed by Ras2, while inactivation of Ras2 resulted in an enhanced degradation of peroxisomes, suggesting a role of Ras2 in the inhibition of peroxisome degradation in glucose-grown cells.