And Cytosolic Proteases Endoplasmic Reticulum Aminopeptidase 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Endomembrane System and Proteins
Chapter 4 | Cell Structure 121 Endosymbiosis We have mentioned that both mitochondria and chloroplasts contain DNA and ribosomes. Have you wondered why? Strong evidence points to endosymbiosis as the explanation. Symbiosis is a relationship in which organisms from two separate species depend on each other for their survival. Endosymbiosis (endo- = “within”) is a mutually beneficial relationship in which one organism lives inside the other. Endosymbiotic relationships abound in nature. We have already mentioned that microbes that produce vitamin K live inside the human gut. This relationship is beneficial for us because we are unable to synthesize vitamin K. It is also beneficial for the microbes because they are protected from other organisms and from drying out, and they receive abundant food from the environment of the large intestine. Scientists have long noticed that bacteria, mitochondria, and chloroplasts are similar in size. We also know that bacteria have DNA and ribosomes, just like mitochondria and chloroplasts. Scientists believe that host cells and bacteria formed an endosymbiotic relationship when the host cells ingested both aerobic and autotrophic bacteria (cyanobacteria) but did not destroy them. Through many millions of years of evolution, these ingested bacteria became more specialized in their functions, with the aerobic bacteria becoming mitochondria and the autotrophic bacteria becoming chloroplasts. The Central Vacuole Previously, we mentioned vacuoles as essential components of plant cells. If you look at Figure 4.8b, you will see that plant cells each have a large central vacuole that occupies most of the cell's area. The central vacuole plays a key role in regulating the cell’s concentration of water in changing environmental conditions. -

Reflux of Endoplasmic Reticulum Proteins to the Cytosol Yields Inactivation of Tumor Suppressors
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.13.038935; this version posted April 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Reflux of Endoplasmic Reticulum proteins to the cytosol yields inactivation of tumor suppressors Daria Sicari1,2, Raphael Pineau1,2, Pierre-Jean Le Reste1,2,3, Luc Negroni4,5,6,7, Sophie Chat8, Aiman Mohtar9, Daniel Thomas8, Reynald Gillet8, M. Ted Hupp9,10, Eric Chevet1,2* and Aeid Igbaria1,2,11* 1Inserm U1242, University of Rennes, Rennes, France. 2Centre de lutte contre le cancer Eugène Marquis, Rennes, France. 3Neurosurgery Dept, University Hospital of Rennes, 35000 Rennes, France. 4Institut de Génétique et de Biologie Moléculaire et Cellulaire, 67404 Illkirch, France. 5Centre National de la Recherche Scientifique, UMR7104, 67404 Illkirch, France. 6Institut National de la Santé et de la Recherche Médicale, U1258, 67404 Illkirch, France. 7Université de Strasbourg, 67404 Illkirch, France. 8Univ. Rennes, CNRS, Institut de Génétique et Développement de Rennes (IGDR) UMR6290, 35000 Rennes, France. 9Edinburgh Cancer Research Centre at the Institute of Genetics and Molecular Medicine, Edinburgh University, Edinburgh, UK. 10International Centre for Cancer Vaccine Science, Gdansk, Poland. 11Department of Life Sciences, Ben-Gurion University of the Negev, Beer Sheva 8410501, Israel. *Correspondence: [email protected], or [email protected] ABSTRACT: In the past decades many studies reported Endoplasmic Reticulum (ER) resident proteins to localize to the cytosol but the mechanisms by which this occurs and whether these proteins exert cytosolic functions remain unknown. We found that select ER luminal proteins accumulate in the cytosol of glioblastoma cells isolated from mouse and human tumors. -

ER-Phagy at a Glance Paolo Grumati1,*, Ivan Dikic1,2,‡ and Alexandra Stolz2,*
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs217364. doi:10.1242/jcs.217364 CELL SCIENCE AT A GLANCE ER-phagy at a glance Paolo Grumati1,*, Ivan Dikic1,2,‡ and Alexandra Stolz2,* ABSTRACT function in response to ER stress signals. This task sharing reflects Selective autophagy represents the major quality control mechanism the complexity of the ER in terms of biological functions and that ensures proper turnover of exhausted or harmful organelles, morphology. In this Cell Science at a Glance article and the among them the endoplasmic reticulum (ER), which is fragmented accompanying poster, we summarize the most recent findings and delivered to the lysosome for degradation via a specific type of about ER-phagy in yeast and in mammalian cells. autophagy called ER-phagy. The recent discovery of ER-resident KEY WORDS: Autophagy, CCPG1, FAM134B, RTN3, SEC62, proteins that bind to mammalian Atg8 proteins has revealed that the Endoplasmic reticulum selective elimination of ER involves different receptors that are specific for different ER subdomains or ER stresses. FAM134B (also known as RETREG1) and RTN3 are reticulon-type proteins that are Introduction able to remodel the ER network and ensure the basal membrane The endoplasmic reticulum (ER) is the largest membrane-bound turnover. SEC62 and CCPG1 are transmembrane ER receptors that organelle in eukaryotic cells. Its complex morphology, which involves sheets, tubules and matrices (Chen et al., 2013; Friedman and Voeltz, 2011; Nixon-Abell et al., 2016), mirrors its diverse roles 1Institute of Biochemistry II, Goethe University Frankfurt - Medical Faculty, in a variety of physiological processes including autophagy University Hospital, 60590 Frankfurt am Main, Germany. -
Endomembrane System
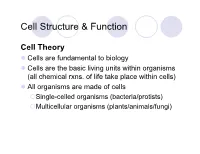
Cell Structure & Function Cell Theory Cells are fundamental to biology Cells are the basic living units within organisms (all chemical rxns. of life take place within cells) All organisms are made of cells Single-celled organisms (bacteria/protists) Multicellular organisms (plants/animals/fungi) Cell Structure & Function Basic Aspects of Cell Structure & Function Plasma membrane Lipid bilayer Proteins DNA-containing region Cytoplasm Eukaryotic v. Prokaryotic cells Prokaryotic v. Eukaryotic Cells Two major classes of cells Prokaryotic cells (pro-, “before”) Cell lacks a “true” nucleus DNA is coiled in a nucleoid region Cells lack nuclear membrane Prokaryotic v. Eukaryotic Cells [attachment structure] [DNA location] [organelles that synthesize proteins] [enclosing the cytoplasm] [rigid structure outside the p.m. ] [jelly-like outer coating] [locomotion organelle] Prokaryotic v. Eukaryotic Cells Eukaryotic cells (eu-, “true”) Nucleus contains most of the cells nuclear material, DNA usually the largest organelle Bordered by a membranous envelope Prokaryotic v. Eukaryotic Cells Plant v. Animal Cells Both contain Plasma membrane (functions as a selective barrier) Nucleus (gene-containing organelle) Cytoplasm (region between nucleus and p.m.) Consists of organelles in a fluid (cytosol) Prokaryotic v. Eukaryotic Cells Plant v. Animal Cells Organelles Bordered by internal membranes Compartmentalizes the functions of a cell Maintains organelle’s unique environment Most organelles are found in both plant and animal cells Plant v. Animal Cells -

ER-Phagy and Its Role in ER Homeostasis in Plants
plants Review ER-Phagy and Its Role in ER Homeostasis in Plants Yan Bao 1,2,* and Diane C. Bassham 1,* 1 Department of Genetics, Development and Cell Biology, Iowa State University, Ames, IA 50011, USA 2 Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI 48824, USA * Correspondence: [email protected] (Y.B.); [email protected] (D.C.B.) Received: 19 November 2020; Accepted: 11 December 2020; Published: 14 December 2020 Abstract: The endoplasmic reticulum (ER) is the largest continuous membrane-bound cellular organelle and plays a central role in the biosynthesis of lipids and proteins and their distribution to other organelles. Autophagy is a conserved process that is required for recycling unwanted cellular components. Recent studies have implicated the ER as a membrane source for the formation of autophagosomes, vesicles that transport material to the vacuole during autophagy. When unfolded proteins accumulate in the ER and/or the ER lipid bilayer is disrupted, a condition known as ER stress results. During ER stress, ER membranes can also be engulfed through autophagy in a process termed ER-phagy. An interplay between ER stress responses and autophagy thus maintains the functions of the ER to allow cellular survival. In this review, we discuss recent progress in understanding ER-phagy in plants, including identification of regulatory factors and selective autophagy receptors. We also identify key unanswered questions in plant ER-phagy for future study. Keywords: autophagy; endoplasmic reticulum; ER stress; ER-phagy; unfolded protein response 1. Introduction Plants live in a world of ever-changing conditions; for survival, they need to adapt to the challenges of their surroundings to balance growth and stress responses [1,2]. -

A Signal-Anchor Sequence Stimulates Signal Recognition Particle Binding to Ribosomes from Inside the Exit Tunnel
A signal-anchor sequence stimulates signal recognition particle binding to ribosomes from inside the exit tunnel Uta Berndta,b,1, Stefan Oellerera,b,c,1, Ying Zhanga,b,c, Arthur E. Johnsond, and Sabine Rosperta,b,2 aInstitute of Biochemistry and Molecular Biology and bCenter for Biological Signalling Studies, University of Freiburg, Stefan-Meier-Strasse 17, D-79104 Freiburg, Germany; cFakulta¨t fu¨ r Biologie, University of Freiburg, Scha¨nzlestrasse 1, D-79104 Freiburg, Germany; and dDepartment of Molecular and Cellular Medicine, Texas A&M Health Science Center, 116 Reynolds Medical Building, College Station, TX 77843 Edited by Arthur Horwich, Yale University School of Medicine, New Haven, CT, and approved December 15, 2008 (received for review August 29, 2008) Sorting of eukaryotic membrane and secretory proteins depends direct interaction between SRP and the nascent chain. Previous on recognition of ribosome-bound nascent chain signal sequences studies have addressed the question of whether or not specific by the signal recognition particle (SRP). The current model suggests amino acid sequences of segments inside the tunnel can further that the SRP cycle is initiated when a signal sequence emerges from the affinity of SRP for RNCs (9, 10). Because signal sequences the ribosomal tunnel and binds to SRP. Then elongation is slowed would be prime candidates for such effects, this possibility was until the SRP-bound ribosome–nascent chain complex (RNC) is tested in the eukaryotic system by using RNCs carrying prep- targeted to the SRP receptor in the endoplasmic reticulum (ER) rolactin, a secreted protein with a cleavable signal sequence. membrane. The RNC is then transferred to the translocon, SRP is However, when nascent preprolactin was too short to exit the released, and translation resumes. -

CELL SNAP Information
CELL SNAP Information Chromosome Lysosome Smooth endoplasmic reticulum Mitochondrion Nucleus DNA Centriole Ribosome Rough endoplasmic reticulum Golgi apparatus Nucleus The nucleus is the largest of the organelles and is at the centre of the cell. It is the storage site of the cell’s DNA. Here DNA is copied and processed into RNA by transcription. This is the first step in the process of protein synthesis. The nucleus is surrounded by a double membrane system called the nuclear envelope that contains holes known as nuclear pores. These pores allow large molecules such as proteins to pass in and out of the nucleus. Inside the nucleus is a round structure known as the nucleolus. This is where ribosomes are formed. Chromosomes Chromosomes are thread-like structures, made of DNA wrapped around a scaffold of proteins. Each human cell contains 46 chromosomes. 23 are passed down from the mother and 23 are passed down from the father. Each chromosome contains specific regions of DNA, known as genes. These are sections of DNA that carry information required to make a molecule, usually a protein. A human has between 23,000 and 25,000 genes. We talk about genes having different characteristics. For instance, if you hear about ‘genes for eye colour’, it means that these genes code for protein pigments in the iris of each of our eyes. 1/3 yourgenome.org CELL SNAP Information DNA DNA, or deoxyribonucleic acid, is a molecule that contains our genetic instructions. It is made up of four chemical bases: adenine (A), cytosine (C), guanine (G) and thymine (T). -

Autophagy Is Activated for Cell Survival After Endoplasmic Reticulum Stress
University of Massachusetts Medical School eScholarship@UMMS Program in Gene Function and Expression Publications and Presentations Molecular, Cell and Cancer Biology 2006-10-13 Autophagy is activated for cell survival after endoplasmic reticulum stress Maiko Ogata University of Miyazaki Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/pgfe_pp Part of the Genetics and Genomics Commons Repository Citation Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. (2006). Autophagy is activated for cell survival after endoplasmic reticulum stress. Program in Gene Function and Expression Publications and Presentations. https://doi.org/10.1128/MCB.01453-06. Retrieved from https://escholarship.umassmed.edu/pgfe_pp/ 113 This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Program in Gene Function and Expression Publications and Presentations by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. MOLECULAR AND CELLULAR BIOLOGY, Dec. 2006, p. 9220–9231 Vol. 26, No. 24 0270-7306/06/$08.00ϩ0 doi:10.1128/MCB.01453-06 Copyright © 2006, American Society for Microbiology. All Rights Reserved. Autophagy Is Activated for Cell Survival after Endoplasmic Reticulum Stressᰔ Maiko Ogata,1,2† Shin-ichiro Hino,1† Atsushi Saito,1,2 Keisuke Morikawa,2 Shinichi Kondo,1 Soshi Kanemoto,1,2 Tomohiko Murakami,1,2 Manabu Taniguchi,3 Ichiro Tanii,1 Kazuya Yoshinaga,1 Sadao Shiosaka,2 James A. -

Lysosomes, Smooth Endoplasmic Reticulum, Mitochondria, And
Undergraduate – Graduate 4. Lysosomes, Smooth Histology Lecture Series Endoplasmic Reticulum, Larry Johnson, Professor Veterinary Integrative Biosciences Mitochondria, and Inclusions Texas A&M University College Station, TX 77843 Objective Lysosomal ultrastructure and function/dysfunction along with continued discussion on protein sorting and protein targeting Smooth endoplasmic reticulum ultrastructure and function in typical cells and those specialized to secrete steroids Mitochondrial ultrastructure, function, origin, and incorporation of cytoplasmic proteins Inclusions Ribosomes translate mRNA in the production of protein. SER Reactions • Scaler reactions Cytosolic proteins a + b = c • Vectorial reactions a + b = c membranes RER proteins • Cytosol is the part of the cytoplasm that is not held by any of the organelles in the cell. On the other hand, cytoplasm is the part of the cell which is contained within the entire cell membrane. • Cytoplasm is cytosol plus organelles = every thing between the cell membrane and the nuclear envelope Cytosolic proteins RER proteins Scaler reactions Vectorial reactions Lysosome Ultrastructure Secondary lysosomes Lysosome Enzymes present - phosphatases, proteases, nucleases, lipid degrading enzymes Lysosome Method of detection - localization of enzymes as primary lysosomes look like secretory granules Histochemical reaction using the local enzyme plus substrate to produce black precititate Localization of lysosomal enzymes Lysosome Negative charges on inner leaflet of lysosomal membrane - protect from -

Immunoelectron-Microscopic Studies of Endoplasmic Reticulum-Golgi Relationships in the Intracellular Transport Process of Lipoprotein Particles in Rat Hepatocytes
J. Cell Sci. 39, 273-290 (1979) 273 Printed in Great Britain © Company of Biologists Limited IMMUNOELECTRON-MICROSCOPIC STUDIES OF ENDOPLASMIC RETICULUM-GOLGI RELATIONSHIPS IN THE INTRACELLULAR TRANSPORT PROCESS OF LIPOPROTEIN PARTICLES IN RAT HEPATOCYTES SHIRO MATSUURA AND YUTAKA TASHIRO* Department of Pliysiology, Kansai Medical University, 1 Fumizonocho, Moriguchi-shi, Osaka 570, Japan SUMMARY Endoplasmic reticulum (ER)-Golgi relationships in the intracellular transport process of secretory proteins in rat hepatocytes have been studied using lipoprotein particles as a marker for the secretory protein and cytochrome P-450 as a marker enzyme for the ER membranes. Ferritin immunoelectron-microscopic observation revealed that, while almot-t all the micro- somal vesicles derived from ER membranes are heavily labelled with ferritin anti-cytochrome P-450 antibody conjugates, labelling of the small peripheral vesicles containing lipoprotein particles, the stacks of Golgi saccules, especially the outermost saccule which is sometimes fenestrated, condensing vacuoles in the trans-Golgi region and the secretion droplets of lipo- protein were scanty and at the control level. Such a characteristic pattern of labelling was especially evident when these structures were prepared from phenobarbital-treated rats. These findings indicate that the membranes of the small peripheral vesicles do not contain cytochrome P-450 and that the cytochrome is probably not transferred to Golgi saccules in the transport process of lipoprotein from ER to Golgi. It is suggested, therefore, that the small peripheral vesicles are formed by budding of the special regions of ER membrane where micro- somal marker proteins such as cytochrome P-450 are excluded and the membrane proteins destined to the Golgi complexes are clustered. -

The Neuronal Endomembrane System III
0270.6474/85/0512-3135$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 5, No. 12. pp. 3135-3144 Pnnted in U.S.A. December 1985 The Neuronal Endomembrane System III. The Origins of Axoplasmic Reticulum Discrete Axonal Cisternae the Axon Hillock’ JAMES D. LINDSEY2 AND MARK H. ELLISMAN Department of Neurosciences, University of California, San Diego, School of Medicine, La Jolla, California 92093 Abstract The axoplasmic reticulum (AR) and the discrete element vesicles were usually found in close association with the (e.g., vesicles, vesiculotubular bodies, multivesicular bodies, trans face of the Golgi apparatus. These results indirectly etc.) constitute the endomembrane system of the axon. It is support the hypothesis that vectors of fast axonal transport, reported here that the AR of bullfrog sciatic nerve readily fills namely the discrete elements, form directly at the trans face with osmium deposits during osmium impregnation. In con- of the Golgi apparatus. From here they move toward and trast, the discrete elements and mitochondria are highly subsequently down the axon without any membrane fission- resistant to impregnation. Hence this preparation is well fusion events with either RER or AR. AR, although it forms suited to address the nature of possible interactions between continuities with RER, retains a distinctly different chemical AR and rough endoplasmic reticulum (RER) in the axon composition from RER as evidenced by its much higher hillock. It is also ideal to study the origin of the axonal discrete affinity for osmium. Thus, it should be considered as an elements within the cell body as well as their interaction with endomembrane component separate from, although inti- other somal endomembrane system components. -

I-Viii Cshperspect-ERT-FM 1..8
This is a free sample of content from The Endoplasmic Reticulum. Click here for more information or to buy the book. The Endoplasmic Reticulum A subject collection from Cold Spring Harbor Perspectives in Biology © 2013 by Cold Spring Harbor Laboratory This is a free sample of content from The Endoplasmic Reticulum. Click here for more information or to buy the book. OTHER SUBJECT COLLECTIONS FROM COLD SPRING HARBOR PERSPECTIVES IN BIOLOGY Wnt Signaling Protein Synthesis and Translational Control The Synapse Extracellular Matrix Biology Protein Homeostasis Calcium Signaling The Golgi Germ Cells The Mammary Gland as an Experimental Model The Biology of Lipids: Trafficking, Regulation, and Function Auxin Signaling: From Synthesis to Systems Biology The Nucleus Neuronal Guidance: The Biology of Brain Wiring Cell Biology of Bacteria Cell–Cell Junctions Generation and Interpretation of Morphogen Gradients Immunoreceptor Signaling NF-kB: A Network Hub Controlling Immunity, Inflammation, and Cancer Symmetry Breaking in Biology The Origins of Life The p53 Family SUBJECT COLLECTIONS FROM COLD SPRING HARBOR PERSPECTIVES IN MEDICINE Addiction Parkinson’s Disease Type 1 Diabetes Angiogenesis: Biology and Pathology HIV: From Biology to Prevention and Treatment The Biology of Alzheimer Disease © 2013 by Cold Spring Harbor Laboratory This is a free sample of content from The Endoplasmic Reticulum. Click here for more information or to buy the book. The Endoplasmic Reticulum A subject collection from Cold Spring Harbor Perspectives in Biology EDITED BY Susan Ferro-Novick Tom A. Rapoport University of California San Diego Harvard Medical School Randy Schekman University of California at Berkeley COLD SPRING HARBOR LABORATORY PRESS Cold Spring Harbor, New York † www.cshlpress.org © 2013 by Cold Spring Harbor Laboratory This is a free sample of content from The Endoplasmic Reticulum.