The Effects of Stream Restoration on Woody Riparian Vegetation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Haas Halo Hydrangea
Out in the Garden Rockport Garden Club, May 2021 What alternatives to harmful insecticides and The Garden Diary: pesticides are available to us? What’s Bugging Stop bugs BEFORE they become a problem: You? 1. Clean up weeds and standing water in your yard which host insects. Did you know there are 200,000,000 insects for every man, woman, and child on earth? Yes, 2. Keep your plants healthy. A healthy plant that is 200 million for each of us! Insects will has its own defenses against many predators. always outnumber us. That is the bad news. 3. Don’t over-fertilize. Too much fertilizer cre- ates weak growth which attracts insects. The good news is that most bugs are either bene- ficial or benign, having no noticeable impact on 4. Be sure plants receive adequate water. Too our lives. We rarely give the good bugs credit little water stresses plants and attracts in- for the work they do. Bees and butterflies polle- sects. nate our plants. Tiny parasitic wasps lay eggs on 5. If bugs are large enough to hand pick, squish larger in- sects and them or put them in a jar of soapy water. kill them 6. Use a garden hose to spray off other insects. in the pro- 7. Create an oasis for birds and butterflies cess. Pray- ing mantis- since birds and other bugs are the worst ene- es kill bee- mies of bad bugs. tles and spiders in Ultimately you may need to use a pesticide. Opt large num- for an organic product whenever possible. -

Retail Plant List by Scientific Name
1404 Citico Rd. Vonore, TN 37885 423.295.2288 office 423.295.2252 fax www.overhillgardens.com 423-295-5003 Avi 423-836-8242 Eileen [email protected] Retail Plant List by Scientific Name Latin Name Common Name Size Price Acer leucoderme Chalk Maple 10 gal $95.00 Acer negundo Boxelder Maple qt+ $16.00 Acer pensylvanicum Striped Maple 2 gal $30.00 Achillea millefolium White Yarrow qt $10.00 Achillea millefolium 'Paprika' Paprika Yarrow qt+ $12.00 Acmella oppositifolia Oppositeleaf Spotflower gal $12.00 Acorus americanus American Sweet Flag qt+ $11.00 Adiantum pedatum Maidenhair Fern gal+ $18.00 Aesculus flava Yellow Buckeye 2 gal $25.00 Aesculus parviflora Bottlebrush Buckeye 3 gal $28.00 Aesculus pavia Red Buckeye gal $18.00 Agarista populifolia (syn. Leucothoe populifolia) Florida Leucothoe 2 gal $25.00 Agastache rupestris Threadleaf Giant Hyssop qt+ $15.00 Aletris farinosa Colic Root qt+ $16.00 Alisma subcordatum American Water Plantain gal+ $16.00 Allium cernuum Nodding Onion qt $10.00 Allium tricoccum Ramps qt $14.00 Alnus incana Speckled Alder 3 gal $28.00 Alnus serrulata Tag Alder 3 gal $25.00 Amelanchier arborea Downy Serviceberry 25/band $15.00 Amelanchier laevis Allegheny Serviceberry 2 gal $25.00 Amelanchier sanguinea Roundleaf Serviceberry 2 gal $30.00 Amelanchier x grandiflora Serviceberry gal $18.00 Amorpha canescens Downy False Indigo gal $16.00 Amorpha fruticosa False Indigo 3 gal $25.00 Amorpha herbacea Hairy False Indigo gal+ $20.00 Amorpha nana Dwarf False Indigo gal $16.00 Amorpha ouachitensis Ouachita False Indigo gal+ $20.00 Ampelaster carolinianus (syn. -
Botanical Name Common Name
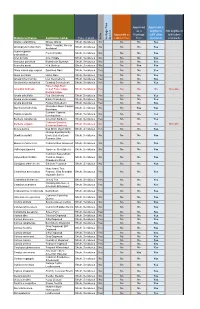
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Floristic Quality Assessment Report
FLORISTIC QUALITY ASSESSMENT IN INDIANA: THE CONCEPT, USE, AND DEVELOPMENT OF COEFFICIENTS OF CONSERVATISM Tulip poplar (Liriodendron tulipifera) the State tree of Indiana June 2004 Final Report for ARN A305-4-53 EPA Wetland Program Development Grant CD975586-01 Prepared by: Paul E. Rothrock, Ph.D. Taylor University Upland, IN 46989-1001 Introduction Since the early nineteenth century the Indiana landscape has undergone a massive transformation (Jackson 1997). In the pre-settlement period, Indiana was an almost unbroken blanket of forests, prairies, and wetlands. Much of the land was cleared, plowed, or drained for lumber, the raising of crops, and a range of urban and industrial activities. Indiana’s native biota is now restricted to relatively small and often isolated tracts across the State. This fragmentation and reduction of the State’s biological diversity has challenged Hoosiers to look carefully at how to monitor further changes within our remnant natural communities and how to effectively conserve and even restore many of these valuable places within our State. To meet this monitoring, conservation, and restoration challenge, one needs to develop a variety of appropriate analytical tools. Ideally these techniques should be simple to learn and apply, give consistent results between different observers, and be repeatable. Floristic Assessment, which includes metrics such as the Floristic Quality Index (FQI) and Mean C values, has gained wide acceptance among environmental scientists and decision-makers, land stewards, and restoration ecologists in Indiana’s neighboring states and regions: Illinois (Taft et al. 1997), Michigan (Herman et al. 1996), Missouri (Ladd 1996), and Wisconsin (Bernthal 2003) as well as northern Ohio (Andreas 1993) and southern Ontario (Oldham et al. -

Natural Vegetation of the Carolinas: Classification and Description of Plant Communities of the Far Western Mountains of North Carolina
Natural vegetation of the Carolinas: Classification and Description of Plant Communities of the Far Western Mountains of North Carolina A report prepared for the Ecosystem Enhancement Program, North Carolina Department of Environment and Natural Resources in partial fulfillments of contract D07042. By M. Forbes Boyle, Robert K. Peet, Thomas R. Wentworth, Michael P. Schafale, and Michael Lee Carolina Vegetation Survey Curriculum in Ecology, CB#3275 University of North Carolina Chapel Hill, NC 27599‐3275 Version 1. April, 2011 1 INTRODUCTION In mid June 2010, the Carolina Vegetation Survey conducted an initial inventory of natural communities along the far western montane counties of North Carolina. There had never been a project designed to classify the diversity of natural upland (and some wetland) communities throughout this portion of North Carolina. Furthermore, the data captured from these plots will enable us to refine the community classification within the broader region. The goal of this report is to determine a classification structure based on the synthesis of vegetation data obtained from the June 2010 sampling event, and to use the resulting information to develop restoration targets for disturbed ecosystems location in this general region of North Carolina. STUDY AREA AND FIELD METHODS From June 13‐20 2010, a total of 48 vegetation plots were established throughout the far western mountains of North Carolina (Figure 1). Focus locations within the study area included the Pisgah National Forest (NF) (French Broad and Pisgah Ranger Districts), the Nantahala NF (Tusquitee Ranger District), and Sandymush Game Land. Target natural communities throughout the week included basic oak‐hickory forest, rich cove forest, northern hardwood and boulderfield forest, chestnut oak forest, montane red cedar woodland, shale slope woodland, montane alluvial slough forest, and low elevation xeric pine forest. -

The Vascular Flora of the Red Hills Forever Wild Tract, Monroe County, Alabama
The Vascular Flora of the Red Hills Forever Wild Tract, Monroe County, Alabama T. Wayne Barger1* and Brian D. Holt1 1Alabama State Lands Division, Natural Heritage Section, Department of Conservation and Natural Resources, Montgomery, AL 36130 *Correspondence: wayne [email protected] Abstract provides public lands for recreational use along with con- servation of vital habitat. Since its inception, the Forever The Red Hills Forever Wild Tract (RHFWT) is a 1785 ha Wild Program, managed by the Alabama Department of property that was acquired in two purchases by the State of Conservation and Natural Resources (AL-DCNR), has pur- Alabama Forever Wild Program in February and Septem- chased approximately 97 500 ha (241 000 acres) of land for ber 2010. The RHFWT is characterized by undulating general recreation, nature preserves, additions to wildlife terrain with steep slopes, loblolly pine plantations, and management areas and state parks. For each Forever Wild mixed hardwood floodplain forests. The property lies tract purchased, a management plan providing guidelines 125 km southwest of Montgomery, AL and is managed by and recommendations for the tract must be in place within the Alabama Department of Conservation and Natural a year of acquisition. The 1785 ha (4412 acre) Red Hills Resources with an emphasis on recreational use and habi- Forever Wild Tract (RHFWT) was acquired in two sepa- tat management. An intensive floristic study of this area rate purchases in February and September 2010, in part was conducted from January 2011 through June 2015. A to provide protected habitat for the federally listed Red total of 533 taxa (527 species) from 323 genera and 120 Hills Salamander (Phaeognathus hubrichti Highton). -

Natural Heritage Program List of Rare Plant Species of North Carolina 2021
Natural Heritage Program List of Rare Plant Species of North Carolina 2021 Compiled by Brenda L. Wichmann, Botanist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1601 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2021 Compiled by Brenda L. Wichmann, Botanist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1601 www.ncnhp.org This list is dynamic and is revised every other year as new data become available. New species are added to the list, and others are dropped from the list as appropriate. Further information may be obtained by contacting the North Carolina Natural Heritage Program, Department of Natural and Cultural Resources, 1651 MSC, Raleigh, NC 27699-1651; by contacting the North Carolina Wildlife Resources Commission, 1701 MSC, Raleigh, NC 27699-1701; or by contacting the North Carolina Plant Conservation Program, Department of Agriculture and Consumer Services, 1060 MSC, Raleigh, NC 27699-1060. -

HKI Risda PGSD FIP Tan Hortensia.Docx
HALAMAN PENGESAHAN Judul : TANAMAN HORTENSIA PENGHARUM RUANGAN NAN ALAMI Peneliti a. Nama Lengkap : Dr. RISDA AMINI, M.P b. NIDN : 0031086303 c. Jabatan Fungsional : Lektor Kepala d. Jurusan : PGSD Fakultas Ilmu Pendidikan e. Perguruan Tinggi : Universitas Negeri Padang f. Alamat surel : [email protected] g. H P : 08126755625 Anggota : - Institusi Mitra : - Biaya : Mandiri Padang, 17 September 2019 Mengetahui: Peneliti Ketua LP2M UNP Prof. Dr. Yasri, M.S Dr. RISDA AMINI, M.P NIP 196303031987031002 NIP 19630831 1989032003 1 Daftar Isi halaman Lembar Pengesahan …………………….………………………………... 1 Daftar Isi ……………………………………………………………… 2 Bab 1 Pendahuluan ……………………………………….……………... 3 1. Latar Belakang ……………………………………..……………….. 3 2. Rumusan Masalah ……………………………………………… 4 Bab 2. METODE PENELITIAN ……………………………………… 5 Bab 3. Pembahasan ……………………………………………………… 7 1. Bunga Kembang Tiga Bulan ……………………………………… 7 2. Perbanyakan ……………………………………………………… 12 3. Syarat Tumbuh ……………………………………………………… 13 4. Morfologi Tanaman ……………………………………………… 14 Bab 4. Implementasi Dalam Kehidupan ……………………………… 15 Bab 5. Penutup ……………………………………………………… 16 Daftar Pustaka ……………………………………………………… 17 2 BAB I PENDAHULUAN a. Latar Belakang Banyak hal penting dalam menjalankan sebuah kehidupan yaitu satu diantaranya berupa interaksi. Makhluk hidup perlu berinteraksi, karena interaksi merupakan suatu aktifitas makhluk hidup untuk dapat berhubungan antara makhluk hidup yang satu dengan yang lain. Salah satu bentuk interaksi yang terjadi dalam kehidupan terutama kita hidup sebagai manusia yang bermasyarakat atau berkeluarga adalah kegiatan bertamu. Tempat yang biasanya digunakan untuk menerima tamu adalah ruang tamu. Selain dari pada itu, jika di ruang teras disediakan kursi dan meja, maka ruang teras juga bisa difungsikan sebagai tempat menerima tamu. Karena terkadang tamu yang datang lebih suka mengobrol di luar (teras) dari pada di dalam (ruang tamu). Disamping itu teras juga suasana lebih santai, udaranya lebih segar dan pandangan mata lebih leluasa. -

100 Years of Change in the Flora of the Carolinas
EUPHORBIACEAE 353 Tragia urticifolia Michaux, Nettleleaf Noseburn. Pd (GA, NC, SC, VA), Cp (GA, SC), Mt (SC): dry woodlands and rock outcrops, particularly over mafic or calcareous rocks; common (VA Rare). May-October. Sc. VA west to MO, KS, and CO, south to FL and AZ. [= RAB, F, G, K, W; = T. urticaefolia – S, orthographic variant] Triadica Loureiro 1790 (Chinese Tallow-tree) A genus of 2-3 species, native to tropical and subtropical Asia. The most recent monographers of Sapium and related genera (Kruijt 1996; Esser 2002) place our single naturalized species in the genus Triadica, native to Asia; Sapium (excluding Triadica) is a genus of 21 species restricted to the neotropics. This conclusion is corroborated by molecular phylogenetic analysis (Wurdack, Hoffmann, & Chase (2005). References: Kruijt (1996)=Z; Esser (2002)=Y; Govaerts, Frodin, & Radcliffe-Smith (2000)=X. * Triadica sebifera (Linnaeus) Small, Chinese Tallow-tree, Popcorn Tree. Cp (GA, NC, SC): marsh edges, shell deposits, disturbed areas; uncommon. May-June; August-November, native of e. Asia. With Euphorbia, Chamaesyce, and Cnidoscolus, one of our few Euphorbiaceous genera with milky sap. Triadica has become locally common from Colleton County, SC southward through the tidewater area of GA, and promises to become a serious weed tree (as it is in parts of LA, TX, and FL). [= K, S, X, Y, Z; = Sapium sebiferum (Linnaeus) Roxburgh – RAB, GW] Vernicia Loureiro 1790 (Tung-oil Tree) A genus of 3 species, trees, native of se. Asia. References: Govaerts, Frodin, & Radcliffe-Smith (2000)=Z. * Vernicia fordii (Hemsley) Airy-Shaw, Tung-oil Tree, Tung Tree. Cp (GA, NC): planted for the oil and for ornament, rarely naturalizing; rare, introduced from central and western China. -

Natural Heritage Program List of Rare Plant Species of North Carolina 2018 Revised October 19, 2018
Natural Heritage Program List of Rare Plant Species of North Carolina 2018 Revised October 19, 2018 Compiled by Laura Gadd Robinson, Botanist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1601 www.ncnhp.org STATE OF NORTH CAROLINA (Wataug>f Wnke8 /Madison V" Burke Y H Buncombe >laywoodl Swain f/~~ ?uthertor< /Graham, —~J—\Jo< Polk Lenoii TEonsylvonw^/V- ^ Macon V \ Cherokey-^"^ / /Cloy Union I Anson iPhmonf Ouptln Scotlar Ons low Robeson / Blodon Ponder Columbus / New>,arrfver Brunewlck Natural Heritage Program List of Rare Plant Species of North Carolina 2018 Compiled by Laura Gadd Robinson, Botanist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1601 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. The list is published every two years. Further information may be obtained by contacting the North Carolina Natural Heritage Program, Department of Natural and Cultural Resources, 1651 MSC, Raleigh, NC 27699-1651; by contacting the North Carolina Wildlife Resources Commission, 1701 MSC, Raleigh, NC 27699- 1701; or by contacting the North Carolina Plant Conservation Program, Department of Agriculture and Consumer Services, 1060 MSC, Raleigh, NC 27699-1060. Additional information on rare species, as well as a digital version of this list, can be obtained from the Natural Heritage Program’s website at www.ncnhp.org. Cover Photo of Allium keeverae (Keever’s Onion) by David Campbell. TABLE OF CONTENTS INTRODUCTION ................................................................................................................. -

Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- FUMARIACEAE
Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- FUMARIACEAE FUMARIACEAE Augustin de Candolle 1821 (Fumitory Family) This family includes 15-20 genera and 500-600 species, herbs, mostly north temperate. The Fumariaceae should likely be merged into the Papaveraceae (Lidén 1981, 1986; Lidén et al. 1997; Judd, Sanders, & Donoghue 1994). References: Stern in FNA (1997); Hill (1992); Lidén (1986, 1981); Lidén et al. (1997); Lidén in Kubitzki, Rohwer, & Bittrich (1993). 1 Corolla with the 2 outer petals spurred or saccate at their bases; [tribe Corydaleae]. 2 Plant a caulescent herbaceous vine (acaulescent in its first year, and appearing to be an herb); ultimate leaf segments 5- 10 mm wide............................................................................. Adlumia 2 Plant an acaulescent herb with basal leaves; ultimate leaf segments 1-4 mm wide. 3 Leaves basal only .................................................................... Dicentra 3 Leaves cauline and basal ....................................................... [Lamprocapnos] 1 Corolla with only 1 outer petal spurred or saccate at its base. 4 Ovary and fruit subglobose, with 1 seed; [tribe Fumarieae] ......................................... Fumaria 4 Ovary and fruit elongate, with several to many seeds; [tribe Corydaleae]. 5 Flowers pink, the petals tipped with yellow; plant biennial; stem erect, 3-8 (-10) dm tall; capsules erect, 25-35 mm long ............................................................................ Capnoides 5 Flowers yellow; plant annual; stem erect, decumbent, or prostrate, 1-3 (-4) dm tall; capsules erect, ascending, divergent, or pendent, 10-20 (-25) mm long................................................ Corydalis Adlumia Rafinesque ex Augustin de Candolle 1821 (Climbing Fumitory) A genus of 2 species, herbs, of e. North America, Korea, and Manchuria. References: Boufford in FNA (1997); Lidén in Kubitzki, Rohwer, & Bittrich (1993). -

Bulletin No. 35: Native Woody Plant Collection Checklist Michael P
Connecticut College Digital Commons @ Connecticut College Bulletins Connecticut College Arboretum 12-1996 Bulletin No. 35: Native Woody Plant Collection Checklist Michael P. Harvey Glenn D. Dreyer Connecticut College Follow this and additional works at: http://digitalcommons.conncoll.edu/arbbulletins Part of the Plant Sciences Commons Recommended Citation Harvey, Michael P. and Dreyer, Glenn D., "Bulletin No. 35: Native Woody Plant Collection Checklist" (1996). Bulletins. Paper 35. http://digitalcommons.conncoll.edu/arbbulletins/35 This Article is brought to you for free and open access by the Connecticut College Arboretum at Digital Commons @ Connecticut College. It has been accepted for inclusion in Bulletins by an authorized administrator of Digital Commons @ Connecticut College. For more information, please contact [email protected]. The views expressed in this paper are solely those of the author. NATIVE WOODY PLANT COLLECTION 0CHECKLIST BULLETIN NO. 35 -THE CONNECTICUT COLLEGE ARBORETUM NEW LONDON, CONNECTICUT CONNECTICUT COLLEGE John C Evans, Chair, Board of Trustees Claire L. Gaudiani '66, President Robert E. Proctor, Provost ARBORETUM STAFF Glenn D. Dreyer MA '83, Director William A. Niering, Research Director Jeffrey D. Smith, Horticulturist Craig O. Vine, Horticultural Assistant Katherine T. Dame '90, Program Coordinator Sally L. Taylor, Education Coordinator Robert A. Askins, Paul E. Fell, Research Associates Pamela G. Hine, MA'84, R. Scott Warren, Research Associates Richard H. Goodwin, Technical Advisor THE CONNECTICUT COLLEGE ARBORETUM ASSOCIATION Membership is open to individuals and organizations interested in supporting the Arboretum and its programs. Members receive Arboretum publications, advance notice of programs and a discount on programs. For more information write to The Connecticut College Arboretum, Campus Box 5201 Conn.