Upregulation of P2RX7 Incx3cr1-Deficient Mononuclear
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cells Δγ Lineage Choice and Shapes Peripheral Purinergic P2X7
Purinergic P2X7 Receptor Drives T Cell Lineage Choice and Shapes Peripheral δγ Cells This information is current as Michela Frascoli, Jessica Marcandalli, Ursula Schenk and of October 2, 2021. Fabio Grassi J Immunol 2012; 189:174-180; Prepublished online 30 May 2012; doi: 10.4049/jimmunol.1101582 http://www.jimmunol.org/content/189/1/174 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2012/05/30/jimmunol.110158 Material 2.DC1 http://www.jimmunol.org/ References This article cites 31 articles, 15 of which you can access for free at: http://www.jimmunol.org/content/189/1/174.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on October 2, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2012 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Purinergic P2X7 Receptor Drives T Cell Lineage Choice and Shapes Peripheral gd Cells Michela Frascoli,* Jessica Marcandalli,* Ursula Schenk,*,1 and Fabio Grassi*,† TCR signal strength instructs ab versus gd lineage decision in immature T cells. -

500 P2RX7 Agonist Treatment Boosts the Ability of IL-12-Activated CD8+ T
J Immunother Cancer: first published as 10.1136/jitc-2020-SITC2020.0500 on 10 December 2020. Downloaded from Abstracts homografts and assessed the relationship between CD5 and metastasis, and deregulation of E-cadherin is a hallmark for increased CD69 and PD-1 (markers of T cell activation and epithelial-mesenchymal transition (EMT). exhaustion) by flow cytometry. Methods The study protocol was approved by the Medical Results We report that T cell CD5 levels were higher in CD4 Ethical Committee of the Academic Medical Centre, Amster- + T cells than in CD8+ T cells in 4T1 tumour-bearing mice, dam, The Netherlands (NL42718.018.12). AIMM’s BCL6 and and that high CD5 levels on CD4+ T cells were maintained Bcl-xL immortalization method1 was used to interrogate the in peripheral organs (spleen and lymph nodes). However, both human antibody repertoire. From a carrier of a pathogenic CD4+ and CD8+ T cells recruited to tumours had reduced gene variant in the MSH6 gene diagnosed with stage IV CRC CD5 compared to CD4+ and CD8+ T cells in peripheral and liver metastasis that had been treated with avastin, capeci- organs. In addition, CD5highCD4+ T cells and CD5highCD8 tabine and oxaliplatin, peripheral-blood memory B cells were + T cells from peripheral organs exhibited higher levels of obtained 9 years after last treatment. Antibodies-containing activation and associated exhaustion compared to CD5lowCD4 supernatant of cultured B-cells were screened for binding to 3 + T cell and CD5lowCD8+ T cell from the same organs. different CRC cell lines (DLD1, LS174T and COLO205) and Interestingly, CD8+ T cells among TILs and downregulated absence of binding to fibroblast by flow cytometry. -

Multifaceted Effects of Extracellular Adenosine Triphosphate and Adenosine in the Tumor–Host Interaction and Therapeutic Perspectives
Multifaceted Effects of Extracellular Adenosine Triphosphate and Adenosine in the Tumor–Host Interaction and Therapeutic Perspectives The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation de Andrade Mello, Paola, Robson Coutinho-Silva, and Luiz Eduardo Baggio Savio. 2017. “Multifaceted Effects of Extracellular Adenosine Triphosphate and Adenosine in the Tumor–Host Interaction and Therapeutic Perspectives.” Frontiers in Immunology 8 (1): 1526. doi:10.3389/fimmu.2017.01526. http://dx.doi.org/10.3389/ fimmu.2017.01526. Published Version doi:10.3389/fimmu.2017.01526 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:34493041 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA REVIEW published: 14 November 2017 doi: 10.3389/fimmu.2017.01526 Multifaceted Effects of Extracellular Adenosine Triphosphate and Adenosine in the Tumor–Host Interaction and Therapeutic Perspectives Paola de Andrade Mello1, Robson Coutinho-Silva 2* and Luiz Eduardo Baggio Savio2* 1 Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 2Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil Cancer is still one of the world’s most pressing health-care challenges, leading to a high number of deaths worldwide. Immunotherapy is a new developing therapy that Edited by: boosts patient’s immune system to fight cancer by modifying tumor–immune cells Salem Chouaib, interaction in the tumor microenvironment (TME). -

Non-Synonymous Single Nucleotide Polymorphisms in the P2X
Int. J. Mol. Sci. 2014, 15, 13344-13371; doi:10.3390/ijms150813344 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Non-Synonymous Single Nucleotide Polymorphisms in the P2X Receptor Genes: Association with Diseases, Impact on Receptor Functions and Potential Use as Diagnosis Biomarkers Emily A. Caseley 1,†, Stephen P. Muench 1, Sebastien Roger 2, Hong-Ju Mao 3, Stephen A. Baldwin 1 and Lin-Hua Jiang 1,4,†,* 1 School of Biomedical Sciences, Faculty of Biological Sciences, University of Leeds, Leeds LS2 9JT, UK; E-Mails: [email protected] (E.A.C.); [email protected] (S.P.M.); [email protected] (S.A.B.) 2 Inserm U1069, University of Tours, Tours 37032, France; E-Mail: [email protected] 3 State Key Laboratory of Transducer Technology, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Science, Shanghai 200050, China; E-Mail: [email protected] 4 Department of Physiology and Neurobiology, Xinxiang Medical University, Xinxiang 453003, China † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +44-0-113-343-4231. Received: 6 June 2014; in revised form: 10 July 2014 / Accepted: 14 July 2014 / Published: 30 July 2014 Abstract: P2X receptors are Ca2+-permeable cationic channels in the cell membranes, where they play an important role in mediating a diversity of physiological and pathophysiological functions of extracellular ATP. Mammalian cells express seven P2X receptor genes. Single nucleotide polymorphisms (SNPs) are widespread in the P2RX genes encoding the human P2X receptors, particularly the human P2X7 receptor. -

Identification of a Subset of Immunosuppressive P2RX1
ARTICLE https://doi.org/10.1038/s41467-020-20447-y OPEN Identification of a subset of immunosuppressive P2RX1-negative neutrophils in pancreatic cancer liver metastasis Xu Wang1,2,4, Li-Peng Hu1,4, Wei-Ting Qin1,4, Qin Yang1,4, De-Yu Chen2,4, Qing Li1, Kai-Xia Zhou1, Pei-Qi Huang1, Chun-Jie Xu1, Jun Li1, Lin-Li Yao1, Ya-Hui Wang1, Guang-Ang Tian1, Jian-Yu Yang3, ✉ ✉ ✉ Min-Wei Yang3, De-Jun Liu3, Yong-Wei Sun 3 , Shu-Heng Jiang 1 , Xue-Li Zhang 1 & ✉ Zhi-Gang Zhang 1 1234567890():,; The immunosuppressive microenvironment that is shaped by hepatic metastatic pancreatic ductal adenocarcinoma (PDAC) is essential for tumor cell evasion of immune destruction. Neutrophils are important components of the metastatic tumor microenvironment and exhibit heterogeneity. However, the specific phenotypes, functions and regulatory mechan- isms of neutrophils in PDAC liver metastases remain unknown. Here, we show that a subset of P2RX1-negative neutrophils accumulate in clinical and murine PDAC liver metastases. RNA sequencing of murine PDAC liver metastasis-infiltrated neutrophils show that P2RX1- deficient neutrophils express increased levels of immunosuppressive molecules, including PD-L1, and have enhanced mitochondrial metabolism. Mechanistically, the transcription factor Nrf2 is upregulated in P2RX1-deficient neutrophils and associated with PD-L1 expression and metabolic reprogramming. An anti-PD-1 neutralizing antibody is sufficient to compromise the immunosuppressive effects of P2RX1-deficient neutrophils on OVA- activated OT1 CD8+ T cells. Therefore, our study uncovers a mechanism by which metastatic PDAC tumors evade antitumor immunity by accumulating a subset of immuno- suppressive P2RX1-negative neutrophils. 1 State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, P.R. -

Increased Gene Copy Number of DEFA1/DEFA3 Worsens Sepsis by Inducing Endothelial Pyroptosis
Increased gene copy number of DEFA1/DEFA3 worsens sepsis by inducing endothelial pyroptosis QiXing Chena, Yang Yangb, JinChao Houb, Qiang Shua, YiXuan Yinc, WeiTao Fuc, Feng Hanc, TingJun Houc,d, CongLi Zengb, Elizabeta Nemethe, Rose Linzmeiere, Tomas Ganze,1, and XiangMing Fanga,b,1 aDepartment of Clinical Research Center, Children’s Hospital, Zhejiang University School of Medicine, 310052 Hangzhou, China; bDepartment of Anesthesiology, The First Affiliated Hospital, Zhejiang University School of Medicine, 310003 Hangzhou, China; cCollege of Pharmaceutical Sciences, Zhejiang University, 310058 Hangzhou, China; dInstitute of Functional Nano and Soft Materials, Soochow University, 215123 Suzhou, China; and eDepartment of Medicine, David Geffen School of Medicine, University of California, Los Angeles, CA 90095 Edited by Michael Zasloff, Georgetown University Medical Center, Washington, DC, and accepted by Editorial Board Member Ruslan Medzhitov December 27, 2018 (received for review July 26, 2018) Sepsis claims an estimated 30 million episodes and 6 million deaths The mechanisms causing the quiescent endothelium to develop per year, and treatment options are rather limited. Human barrier dysfunction during sepsis might hold the key to future neutrophil peptides 1–3 (HNP1–3) are the most abundant neutro- therapeutic strategies, but they are still largely unknown. phil granule proteins but their neutrophil content varies because Defensins are short cationic, amphiphilic, cysteine-rich anti- of unusually extensive gene copy number polymorphism. A ge- microbial peptides with three or four intramolecular disulfide netic association study found that increased copy number of the bonds. They are classified as α-, β-, and θ-defensins, of which the HNP-encoding gene DEFA1/DEFA3 is a risk factor for organ dys- first two are the most common human antimicrobial peptides function during sepsis development. -
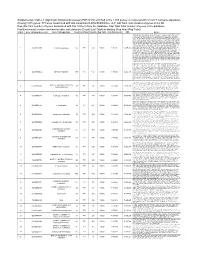
(FDR<0.05) Enriched in the 1015 Genes Co-Clustered with Known T
Supplementary Table 3. Significant biological processes (FDR<0.05) enriched in the 1,015 genes co-clustered with known T cell gene signatures. Among 1,015 genes, 771 were associated with GO annotation in DAVID database v6.7. List Total: total number of genes in my list. Pop Hits: total number of genes associated with this GO term from the database. Pop Total: total number of genes in the database. Fold Enrichment: relative enrichment ratio, calculated by (Count)/(List Total) divided by (Pop Hits)/(Pop Total). Index Gene Ontology Accession Gene Ontology Name Count List Total Pop Hits Pop Total Fold Enrichment FDR Genes AQP9, C1QC, B2M, LILRA1, LILRA2, CLEC4E, S1PR4, LILRA4, IFNG, LILRA6, CLEC4A, VNN1, ERAP2, FAS, CRTAM, C5AR1, GBP5, NCF2, NCF1, NCF4, SERPING1, HLA-DQA2, HLA-DQA1, PDCD1LG2, LILRB1, CCR9, C1QA, C1QB, LILRB2, CCR7, CCR6, UNC13D, CCR5, CD40LG, CCR4, LILRB3, CCR2, LILRB4, HLA-DPA1, VSIG4, HLA-DRA, IL1R2, IL1R1, HLA-DRB1, OAS3, ACP5, OAS1, OAS2, CD74, IFI35, ZAP70, FCER1G, HLA-DRB5, HLA-DPB1, HLA-DOA, HLA-DOB, DHX58, BLNK, IL23R, KIR2DS4, CD300C, SLAMF7, OASL, RGS1, APOL1, CD300A, HMHB1, CD209, CLEC7A, LY86, LY9, CLNK, FCRL4, SH2D1A, NOD2, HAMP, CCL3L1, CCL3L3, TICAM2, ICAM1, GZMA, CMKLR1, LY96, WAS, IL18BP, LAX1, TNFSF12- TNFSF13, HLA-DQB1, CSF2, GPR183, CCR1, GPR65, CXCL9, NCF1C, IL7R, CLEC10A, CCL24, CCL22, CYP27B1, CCL23, FCGR1C, FTHL3, FCGR1A, FCGR1B, BCL3, C2, CD27, CD28, FYB, IL18R1, IL7, CD1C, CTLA4, CCL19, CD1B, CD1A, TRIM22, CD180, CD1E, CCL18, CCL17, CCL13, FCGR2B, FCGR2C, P2RY14, LIME1, CD14, IL16, IL18, TLR1, TNFSF15, -

Anti-P2RX7 / P2X7 Antibody (ARG43366)
Product datasheet [email protected] ARG43366 Package: 50 μg anti-P2RX7 / P2X7 antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes P2RX7 / P2X7 Tested Reactivity Ms Tested Application WB Host Rabbit Clonality Polyclonal Isotype IgG Target Name P2RX7 / P2X7 Species Mouse Immunogen Recombinant protein corresponding to N380-E585 of Mouse P2RX7 / P2X7. Conjugation Un-conjugated Alternate Names ATP receptor; P2X7; P2X purinoceptor 7; P2Z receptor; Purinergic receptor Application Instructions Application table Application Dilution WB 1:500 - 1:2000 Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Positive Control Neuro-2a Observed Size ~ 70 kDa Properties Form Liquid Purification Affinity purification with immunogen. Buffer 0.2% Na2HPO4, 0.9% NaCl, 0.01% Sodium azide and 4% Trehalose. Preservative 0.01% Sodium azide Stabilizer 4% Trehalose Concentration 0.5 mg/ml Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. Note For laboratory research only, not for drug, diagnostic or other use. www.arigobio.com 1/2 Bioinformation Gene Symbol P2RX7 Gene Full Name purinergic receptor P2X, ligand gated ion channel, 7 Background The product of this gene belongs to the family of purinoceptors for ATP. This receptor functions as a ligand-gated ion channel and is responsible for ATP-dependent lysis of macrophages through the formation of membrane pores permeable to large molecules. -

Extracellular ATP Released from Candida Albicans Activates Non-Peptidergic Neurons to Augment Host Defense
bioRxiv preprint doi: https://doi.org/10.1101/2020.01.27.921049; this version posted January 28, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Extracellular ATP released from Candida albicans activates non-peptidergic neurons to augment host defense SHORT TITLE (70 characters): Candida albicans secretes ATP to augment host defense Tara N Edwards¶,1, Shiqun Zhang¶,1, Andrew Liu1, Jonathan A. Cohen1, Paul Yifan Zhou1, Selene Mogavero2, Bernhard Hube2, Judith Berman3, Marie-Elisabeth Bougnoux4,5, Alicia R. Mathers1, Sarah L. Gaffen6, Kathryn M. Albers7,8, H. Richard Koerber7,8, Brian M. Davis7,8, Christophe D'Enfert4, Daniel H Kaplan1,* 1 Departments of Dermatology and Immunology, University of Pittsburgh, Pittsburgh, Pennsylvania 2 Department of Microbial Pathogenicity Mechanisms, Leibniz Institute for Natural Product Research and Infection Biology-Hans Knoell Institute (HKI), Jena, Germany 3 School of Molecular Cell Biology & Biotechnology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Ramat Aviv, Israel 4 Unité Biologie et Pathogénicité Fongiques, Département de Mycologie, Institut Pasteur, USC2019 INRA, Paris, France 5 Unité de Parasitologie-Mycologie, Service de Microbiologie clinique, Hôpital Necker- Enfants-Malades, Assistance Publique des Hôpitaux de Paris (APHP), Paris, France 6 Division of Rheumatology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA 7 Pittsburgh Center for Pain Research, University of Pittsburgh, Pittsburgh, PA 15261, USA 8 Department of Neurobiology, University of Pittsburgh, Pittsburgh, PA 15261, USA * Corresponding author E-mail: [email protected] (DHK) ¶Authors provided equal contribution Keywords: Candida albicans, ATP, peripheral nervous system, sensory neuron, non- peptidergic neuron. -

And Cystein Cathepsin-Dependent Cancer Cells Invasiveness
Oncogene (2011) 30, 2108–2122 & 2011 Macmillan Publishers Limited All rights reserved 0950-9232/11 www.nature.com/onc ORIGINAL ARTICLE P2X7 receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness B Jelassi1, A Chantoˆme1, F Alcaraz-Pe´rez2, A Baroja-Mazo3, ML Cayuela2, P Pelegrin3,4, A Surprenant4 and S Roger1 1Inserm U921, Universite´ Franc¸ois Rabelais de Tours, 10 Boulevard Tonnelle´, Tours, France; 2Telomerase, Cancer and Aging Group, University Hospital ‘Virgen de la Arrixaca’-FFIS, Carretera Palmar, Murcia, Spain; 3Inflammation and Experimental Surgery Group, University Hospital ‘Virgen de la Arrixaca’-FFIS, Carretera Palmar, Murcia, Spain and 4Faculty of Life Science, Michael Smith Building D3315, University of Manchester, Manchester, UK ATP-gated P2X7 receptors (P2X7R) are unusual plasma cancer patients do not die because of local complications membrane ion channels that have been extensively studied of their primary solid tumour growth, which are in immune cells. More recently, P2X7R have been relatively well treated, but rather to the appearance of described as potential cancer cell biomarkers. However, metastases. The development of metastases consists of a mechanistic links between P2X7R and cancer cell complex series of events accomplished by tumour cells processes are unknown. Here, we show, in the highly after they acquire numerous abilities, one of which being aggressive human breast cancer cell line MDA-MB-435s, the acquisition of an invasive potency. This mainly lies that P2X7 receptor is highly expressed and fully into an enhanced migration and the ability to digest the functional. Its activation is responsible for the extension extracellular matrix (Gupta and Massague, 2006). -

Brilliant Blue Dyes in Daily Food: How Could Purinergic System Be Affected?
Hindawi Publishing Corporation International Journal of Food Science Volume 2016, Article ID 7548498, 13 pages http://dx.doi.org/10.1155/2016/7548498 Review Article Brilliant Blue Dyes in Daily Food: How Could Purinergic System Be Affected? Leonardo Gomes Braga Ferreira,1 Robson Xavier Faria,2 Natiele Carla da Silva Ferreira,3 and Rômulo José Soares-Bezerra3 1 Laboratory of Inflammation, Oswaldo Cruz Foundation, Av. Brazil, 4365 Rio de Janeiro, RJ, Brazil 2Laboratory of Toxoplasmosis, Oswaldo Cruz Foundation, Av. Brazil, 4365 Rio de Janeiro, RJ, Brazil 3Laboratory of Cellular Communication, Oswaldo Cruz Foundation, Av. Brazil, 4365 Rio de Janeiro, RJ, Brazil Correspondence should be addressed to Robson Xavier Faria; [email protected] Received 31 May 2016; Accepted 28 August 2016 Academic Editor: Rosana G. Moreira Copyright © 2016 Leonardo Gomes Braga Ferreira et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Dyes were first obtained from the extraction of plant sources in the Neolithic period to produce dyed clothes. At the beginning ofthe 19th century, synthetic dyes were produced to color clothes on a large scale. Other applications for synthetic dyes include the phar- maceutical and food industries, which are important interference factors in our lives and health. Herein, we analyzed the possible implications of some dyes that are already described as antagonists of purinergic receptors, including special Brilliant Blue G and its derivative FD&C Blue No. 1. Purinergic receptor family is widely expressed in the body and is critical to relate to much cellular homeostasis maintenance as well as inflammation and cell death. -
Cells Δγ Lineage Choice and Shapes Peripheral Purinergic P2X7
Purinergic P2X7 Receptor Drives T Cell Lineage Choice and Shapes Peripheral δγ Cells This information is current as Michela Frascoli, Jessica Marcandalli, Ursula Schenk and of September 29, 2021. Fabio Grassi J Immunol 2012; 189:174-180; Prepublished online 30 May 2012; doi: 10.4049/jimmunol.1101582 http://www.jimmunol.org/content/189/1/174 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2012/05/30/jimmunol.110158 Material 2.DC1 http://www.jimmunol.org/ References This article cites 31 articles, 15 of which you can access for free at: http://www.jimmunol.org/content/189/1/174.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 29, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2012 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Purinergic P2X7 Receptor Drives T Cell Lineage Choice and Shapes Peripheral gd Cells Michela Frascoli,* Jessica Marcandalli,* Ursula Schenk,*,1 and Fabio Grassi*,† TCR signal strength instructs ab versus gd lineage decision in immature T cells.