Paleoclimate Variations and Impact on Groundwater
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE DEVELOPMENT of MESOZOIC SEDIMENTARY IBASINS AROUND the MARGINS of the NORTH ATLANTIC and THEIR HYDROCARBON POTENTIAL D.G. Masson and P.R
INTERNAL DOCUMENT IIL THE DEVELOPMENT OF MESOZOIC SEDIMENTARY IBASINS AROUND THE MARGINS OF THE NORTH ATLANTIC AND THEIR HYDROCARBON POTENTIAL D.G. Masson and P.R. Miles Internal Document No. 195 December 1983 [This document should not be cited in a published bibliography, and is supplied for the use of the recipient only]. iimsTit\jte of \ OCEANOGRAPHIC SCIENCES % INSTITUTE OF OCEANOGRAPHIC SCIENCES Wormley, Godalming, Surrey GU8 5UB (042-879-4141) (Director: Dr. A. S. Laughton, FRS) Bidston Observatory, Crossway, Birkenhead, Taunton, Mersey side L43 7RA Somerset TA1 2DW (051-653-8633) (0823-86211) (Assistant Director: Dr. D. E. Cartwright) (Assistant Director: M.J. Tucker) THE DEVELOPMENT OF MESOZOIC SEDIMENTARY BASINS AROUND THE MARGINS OF THE NORTH ATLANTIC AND THEIR HYDROCARBON POTENTIAL D.G. Masson and P.R. Miles Internal Document No. 195 December 1983 Work carried out under contract to the Department of Energy This document should not be cited in a published bibliography except as 'personal communication', and is supplied for the use of the recipient only. Institute of Oceanographic Sciences, Brook Road, Wormley, Surrey GU8 SUB ABSTRACT The distribution of Mesozoic basins around the margins of the North Atlantic has been summarised, and is discussed in terms of a new earliest Cretaceous palaeogeographic reconstruction for the North Atlantic area. The Late Triassic-Early Jurassic rift basins of Iberia, offshore eastern Canada and the continental shelf of western Europe are seen to be the fragments of a formerly coherent NE trending rift system which probably formed as a result of tensional stress between Europe, Africa and North America. The separation of Europe, North America and Iberia was preceded by a Late Jurassic-Early Cretaceous rifting phase which is clearly distinct from the earlier Mesozoic rifting episode and was little influenced by it. -

New Micropalaeontological Studies on the Type Section of the Campanian-Maastrichtian at Tercis
New micropalaeontological studies on the type section of the Campanian-Maastrichtian at Tercis (SW France): new ostracodes obtained using acetolysis Bernard Andreu, Gilles Serge Odin To cite this version: Bernard Andreu, Gilles Serge Odin. New micropalaeontological studies on the type section of the Campanian-Maastrichtian at Tercis (SW France): new ostracodes obtained using acetolysis. Carnets de Geologie, Carnets de Geologie, 2008, CG2008 (A02), pp.1-14. hal-00235838v2 HAL Id: hal-00235838 https://hal.archives-ouvertes.fr/hal-00235838v2 Submitted on 21 Mar 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Carnets de Géologie / Notebooks on Geology - Article 2008/02 (CG2008_A02) New micropalaeontological studies on the type section of the Campanian-Maastrichtian at Tercis (SW France): new ostracodes obtained using acetolysis 1 Bernard ANDREU 2 Gilles Serge ODIN Abstract: Through the use of acetolysis new micropalaeontological studies on the type section of the Campanian-Maastrichtian boundary at Tercis (SW France) we obtained additional information on ostracodes. Acetolysis on hard carbonates levels of the Tercis quarry found 22 species in addition to those of the studies published in 2001. Today, 75 species are recognized: they represent 34 known genera, and 4 currently unidentified. -

French Geothermal Resources Survey BRGM Contribution to the Market Study in the LOW-BIN Project
French geothermal resources survey BRGM contribution to the market study in the LOW-BIN project BRGM/RP - 57583 - FR August, 2009 French geothermal resources survey BRGM contribution to the market study in the LOW-BIN project (TREN/05/FP6EN/S07.53962/518277) BRGM/RP-57583-FR August, 2009 F. Jaudin With the collaboration of M. Le Brun, V.Bouchot, C. Dezaye IM 003 ANG – April 05 Keywords: French geothermal resources, geothermal heat, geothermal electricity generation schemes, geothermal Rankine Cycle, cogeneration, geothermal binary plants In bibliography, this report should be cited as follows: Jaudin F. , Le Brun M., Bouchot V., Dezaye C. (2009) - French geothermal resources survey, BRGM contribution to the market study in the LOW-BIN Project. BRGM/RP-57583 - FR © BRGM, 2009. No part of this document may be reproduced without the prior permission of BRGM French geothermal resources survey BRGM contribution to the market study in the LOW-BIN Project BRGM, July 2009 F.JAUDIN M. LE BRUN, V. BOUCHOT, C. DEZAYE 1 / 33 TABLE OF CONTENT 1. Introduction .......................................................................................................................4 2. The sedimentary regions...................................................................................................5 2.1. The Paris Basin ........................................................................................................5 2.1.1. An overview of the exploitation of the low enthalpy Dogger reservoir ..............7 2.1.2. The geothermal potential -
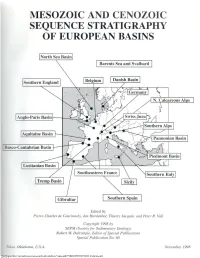
Mesozoic and Cenozoic Sequence Stratigraphy of European Basins
Downloaded from http://pubs.geoscienceworld.org/books/book/chapter-pdf/3789969/9781565760936_frontmatter.pdf by guest on 26 September 2021 Downloaded from http://pubs.geoscienceworld.org/books/book/chapter-pdf/3789969/9781565760936_frontmatter.pdf by guest on 26 September 2021 MESOZOIC AND CENOZOIC SEQUENCE STRATIGRAPHY OF EUROPEAN BASINS PREFACE Concepts of seismic and sequence stratigraphy as outlined in To further stress the importance of well-calibrated chronos- publications since 1977 made a substantial impact on sedimen- tratigraphic frameworks for the stratigraphic positioning of geo- tary geology. The notion that changes in relative sea level shape logic events such as depositional sequence boundaries in a va- sediment in predictable packages across the planet was intui- riety of depositional settings in a large number of basins, the tively attractive to many sedimentologists and stratigraphers. project sponsored a biostratigraphic calibration effort directed The initial stratigraphic record of Mesozoic and Cenozoic dep- at all biostratigraphic disciplines willing to participate. The re- ositional sequences, laid down in response to changes in relative sults of this biostratigraphic calibration effort are summarized sea level, published in Science in 1987 was greeted with great, on eight charts included in this volume. albeit mixed, interest. The concept of sequence stratigraphy re- This volume also addresses the question of cyclicity as a ceived much acclaim whereas the chronostratigraphic record of function of the interaction between tectonics, eustasy, sediment Mesozoic and Cenozoic sequences suffered from a perceived supply and depositional setting. An attempt was made to estab- absence of biostratigraphic and outcrop documentation. The lish a hierarchy of higher order eustatic cycles superimposed Mesozoic and Cenozoic Sequence Stratigraphy of European on lower-order tectono-eustatic cycles. -

A Review of the Late Jurassic–Early Cretaceous Charophytes from The
A review of the Late Jurassic–Early Cretaceous charophytes from the northern Aquitaine Basin in south-west France Roch-Alexandre Benoit, Didier Néraudeau, Carles Martin-Closas To cite this version: Roch-Alexandre Benoit, Didier Néraudeau, Carles Martin-Closas. A review of the Late Jurassic– Early Cretaceous charophytes from the northern Aquitaine Basin in south-west France. Cretaceous Research, Elsevier, 2017, 79, pp.199-213. <10.1016/j.cretres.2017.07.009>. <insu-01574653> HAL Id: insu-01574653 https://hal-insu.archives-ouvertes.fr/insu-01574653 Submitted on 16 Aug 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript A review of the Late Jurassic–Early Cretaceous charophytes from the northern Aquitaine Basin in south-west France Roch-Alexandre Benoit, Didier Neraudeau, Carles Martín-Closas PII: S0195-6671(17)30121-0 DOI: 10.1016/j.cretres.2017.07.009 Reference: YCRES 3658 To appear in: Cretaceous Research Received Date: 13 March 2017 Revised Date: 5 July 2017 Accepted Date: 17 July 2017 Please cite this article as: Benoit, R.-A., Neraudeau, D., Martín-Closas, C., A review of the Late Jurassic–Early Cretaceous charophytes from the northern Aquitaine Basin in south-west France, Cretaceous Research (2017), doi: 10.1016/j.cretres.2017.07.009. -

Brittle Tectonics and Fluids Overpressure During the Early
Brittle tectonics and fluids overpressure during the early stage of the Bay of Biscay opening in the Jard-sur-Mer area, (northern Aquitaine Basin, France) Pierre Strzerzynski, Louise Lenoir, Paul Bessin, Loic Bouat To cite this version: Pierre Strzerzynski, Louise Lenoir, Paul Bessin, Loic Bouat. Brittle tectonics and fluids overpressure during the early stage of the Bay of Biscay opening in the Jard-sur-Mer area, (northern Aquitaine Basin, France). Bulletin de la Société Géologique de France, Société géologique de France, 2020, 191, 10.1051/bsgf/2020025. insu-03231747 HAL Id: insu-03231747 https://hal-insu.archives-ouvertes.fr/insu-03231747 Submitted on 21 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License BSGF - Earth Sciences Bulletin 2020, 191, 38 © P. Strzerzynski et al., Published by EDP Sciences 2020 https://doi.org/10.1051/bsgf/2020025 Available online at: www.bsgf.fr Brittle tectonics and fluids overpressure during the early stage of the Bay of Biscay opening in the Jard-sur-Mer area, (northern Aquitaine Basin, France) Pierre Strzerzynski1,*, Louise Lenoir2, Paul Bessin1 and Loic Bouat1 1 Laboratoire de Planétologie et Géodynamique, UMR 6112, CNRS, Le Mans Université, Avenue Olivier Messiaen, 72085 Le Mans Cedex 9, France 2 UMR CNRS/UPS Géosciences Paris Sud (GEOPS), Bât. -

Deliverable D4.2 Mapping of Potential Heat End- Users Around Vermilion Sites in France
Ref. Ares(2019)2913757 - 30/04/2019 Document ID: D4.2 Mapping of potential heat end-users around Vermilion sites in France H2020 Grant Agreement N° 792037 DELIVERABLE D4.2 MAPPING OF POTENTIAL HEAT END- USERS AROUND VERMILION SITES IN FRANCE WP4: ENHANCING PETROLEUM SEDIMENTARY BASINS FOR GEOTHERMAL ELECTRICITY AND THERMAL POWER PRODUCTION Contractual delivery date: M12 Actual delivery date: M12 PROJECT INFORMATION Grant Agreement n° 792037 Dates 1st May 2018 – 31 October 2021 PROPRIETARY RIGHTS STATEMENT This document contains information, which is proprietary to the MEET consortium. Neither this document nor the information contained herein shall be used, duplicated or communicated by any means to any third party, in whole or in parts, except with prior written consent of the MEET consortium. 1 Version : VX // Dissemination level : X Document ID: D4.2 Mapping of potential heat end-users around Vermilion sites in France H2020 Grant Agreement N° 792037 DOCUMENT INFORMATION Version VF Dissemination level PU Editor SABINE LOR (VERMILION) Other authors DOCUMENT APPROVAL Position in Name Organisation Date Visa project ALBERT GENTER Project ES GEOTHERMIE ELEONORE Coordinator 24/04/2019 OK DALMAIS ERIC LEOUTRE WP4 Leader VERMILION 24/04/2019 OK MARGAUX Project Manager AYMING MAROT Officer 25/04/2019 OK GHISLAIN Scientific Project UNILASALLE 24/04/2019 OK TRULLENQUE Manager Assistant UNIVERSITY OF IVA RASJL 28/04/2019 OK Professor ZAGREB DOCUMENT HISTORY Version Date Modifications Authors V1 19/04/2019 ToC LOR / VERMILION V2 19/04/2019 Main -

High-Temperature Metamorphism During Extreme Thinning of the Continental Crust: a Reappraisal of the North Pyrenean Passive Paleomargin
Solid Earth, 6, 643–668, 2015 www.solid-earth.net/6/643/2015/ doi:10.5194/se-6-643-2015 © Author(s) 2015. CC Attribution 3.0 License. High-temperature metamorphism during extreme thinning of the continental crust: a reappraisal of the North Pyrenean passive paleomargin C. Clerc1,2,*,**, A. Lahfid3, P. Monié2, Y. Lagabrielle4, C. Chopin1, M. Poujol4, P. Boulvais4, J.-C. Ringenbach5, E. Masini5, and M. de St Blanquat6 1Laboratoire de Géologie, CNRS-UMR8538, Ecole normale supérieure, 24 rue Lhomond, 75231 Paris, France 2Géosciences Montpellier, CNRS-UMR 5243, Université de Montpellier 2, Place Eugène Bataillon, 34095 Montpellier, France 3BRGM/ISTO, 3 avenue Claude Guillemin, 45000 Orléans, France 4Géosciences Rennes, UMR 6118, Université Rennes 1, Campus de Beaulieu, 35042 Rennes, France 5TOTAL, CSTJF, avenue Larribau, 64000 Pau, France 6GET, 14 avenue Edouard Belin, 31400 Toulouse, France *now at: ISTO, 1A rue de la Férollerie, 45100 Orléans, France ** Invited contribution by C. Clerc, recipient of the EGU Outstanding Student Poster (OSP) Award 2012. Correspondence to: C. Clerc ([email protected]) Received: 12 January 2015 – Published in Solid Earth Discuss.: 20 February 2015 Revised: 13 April 2015 – Accepted: 16 May 2015 – Published: 8 June 2015 Abstract. An increasing number of field examples in moun- and no westward or eastward propagation of the metamor- tain belts show that the formation of passive margins during phism and magmatism can be clearly identified. In contrast, extreme continent thinning may occur under conditions of the new data reveal a progressive propagation of the ther- high to very high thermal gradient beneath a thin cover of mal anomaly from the base to the surface of the continental syn-rift sediments. -

Geological Resource Analysis of Shale Gas and Shale Oil in Europe
Draft Report for DG JRC in the Context of Contract JRC/PTT/2015/F.3/0027/NC "Development of shale gas and shale oil in Europe" European Unconventional Oil and Gas Assessment (EUOGA) Geological resource analysis of shale gas and shale oil in Europe Deliverable T4b mmmll Geological resource analysis of shale gas/oil in Europe June 2016 I 2 Geological resource analysis of shale gas/oil in Europe Table of Contents Table of Contents .............................................................................................. 3 Abstract ........................................................................................................... 6 Executive Summary ........................................................................................... 7 Introduction ...................................................................................................... 8 Item 4.1 Setup and distribute a template for uniformly describing EU shale plays to the National Geological Surveys .........................................................................12 Item 4.2 Elaborate and compile general and systematic descriptions of the shale plays from the NGS responses ....................................................................................15 T01, B02 - Norwegian-Danish-S. Sweden – Alum Shale .........................................16 T02 - Baltic Basin – Cambrian-Silurian Shales ......................................................22 T03 - South Lublin Basin, Narol Basin and Lviv-Volyn Basin – Lower Paleozoic Shales ......................................................................................................................37 -

Stratigraphic and Tectonic Studies in the Central Aquitaine Basin, Northern Pyrenees: Constraints on the Subsidence and Deformat
C. R. Geoscience 348 (2016) 224–235 Contents lists available at ScienceDirect Comptes Rendus Geoscience w ww.sciencedirect.com Tectonics, Tectonophysics Stratigraphic and tectonic studies in the central Aquitaine Basin, northern Pyrenees: Constraints on the subsidence and deformation history of a retro-foreland basin a a,b, c Ge´raldine Rougier , Mary Ford *, Fre´de´ric Christophoul , d Anne-Gae¨lle Bader a Universite´ de Lorraine, ENSG, INP, rue du Doyen-Marcel-Roubault, 54501 Vandœuvre-le`s-Nancy, France b CRPG, UMR 7358, 15, rue Notre-Dame-des-Pauvres, 54501 Vandœuvre-le`s-Nancy, France c GET, UMR 5563, Universite´ de Toulouse, CNRS–IRD–OMP, 14, avenue E´douard-Belin, 31400 Toulouse, France d BRGM, 3, avenue Claude-Guillemin, 45060 Orle´ans, France A R T I C L E I N F O A B S T R A C T Article history: The central North-Pyrenean retrowedge developed on a thinned lithosphere, rich in Received 17 December 2015 Keuper evaporites. The behavior of this retro-foreland system is studied using subsidence Accepted after revision 18 December 2015 analyses and a sequentially restored cross-section (120 km, Saint-Gaudens to Castelsar- Available online 24 March 2016 rasin) constrained by new chrono- and lithostratigraphy, surface and subsurface data. During the Late Cretaceous, a first episode of foreland subsidence (E1) produced a narrow Handled by Michel de Saint Blanquat marine depocenter (Comminges Basin, 30 km wide), supplied from the east. A synchronous early deformation involved inversion of basement faults and gentle Keywords: shortening (4.5 km) of the Mesozoic strata above a Keuper decoupling layer. -

Fluvial Evolution of the Garonne River, France
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2005 Fluvial evolution of the Garonne River, France: integrating field ad ta with numerical simulations Robin Rene Lancaster Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Earth Sciences Commons Recommended Citation Lancaster, Robin Rene, "Fluvial evolution of the Garonne River, France: integrating field data with numerical simulations" (2005). LSU Master's Theses. 3351. https://digitalcommons.lsu.edu/gradschool_theses/3351 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. FLUVIAL EVOLUTION OF THE GARONNE RIVER, FRANCE: INTEGRATING FIELD DATA WITH NUMERICAL SIMULATIONS A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in The Department of Geology & Geophysics by Robin Rene Lancaster B.S., Colorado State University, 2003 December 2005 ACKNOWLEDGMENTS This project could not have been completed without the invaluable help of many people, and I would like to take this opportunity to acknowledge that assistance. I would first like to thank my Advisor, Dr. Michael Blum, who provided support and guidance during every step of this project, from its inception to its completion. Always patient, insightful, supportive, and brilliantly intelligent, I hold Dr. -

Aquitaine Coast (France)
EUROSION Case Study AQUITAINE COAST (FRANCE) Contact: Jean Christophe LE BRETON Anne GUCHAN Conseil Regional d’Aquitaine 14 Rue François de Sourdis 33077 Bordeaux (CEDEX) Tel: +34 055 757 83 06 10 Fax: +34 055 656 38 06 e-mail: [email protected] [email protected] 1 EUROSION Case Study 1. GENERAL DESCRIPTION OF THE AREA The Aquitaine coast is located in the southwest of France, and forms a vast triangular basin surrounded to the north-east by the hercynian terrains of the Massif Armoricain and the Massif Central, which join with the Pyrenees to the south through the Montagne Noire. 1.1 Physical process level 1.1.1 Classification General: sandy beaches CORINE: beaches Coastal guide: coastal plain The Aquitaine coast is a straight sedimentary coast with a longitudinal dune system, with several lagoons (the largest of which is the Arcachon lagoon). The study area comprises two coastal towns: Lacanau and Cap-Ferret (see Figure1). 1.1.2 Geology Aquitaine is a coastal plain composed of quaternary sediment. The main coastal formations are sand dunes, with the highest dune formation known in Europe. The characteristic sediment of this coast is well sorted sand, of medium to fine grain size (~300µm. The continental shelf forms a wide plateau of the “depositional type”. 1.1.3 Morphology of the coast Along the Atlantic ocean, the 230km-long sandy coast of Aquitaine region (see Figure 1) is limited to the south by the Ardour river and to the north by the mouth of the Gironde estuary issued from the junction between the Garonne and Dordogne rivers which drain respectively the Massif central mountains, and the Pyrenees.