Performance and Emission Analysis of Waste Vegetable Oil on C.I. Engine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Public Works & Infrastructure Committee
Public Works & Infrastructure Committee Geoff Rathbone, General Manager, Solid Waste Management Services Introduction Solid Waste Management Services is responsible for: Managing approximately 1 million tonnes of waste annually Collecting, transfer, processing and disposal of municipal waste Managing waste diversion programs Managing household hazardous waste programs Providing transfer station loading services to private sector customers and the Regional Municipality of Peel Disposal services to York Region, City of Guelph, City of St. Thomas and other Municipalities in vicinity of Green Lane Collecting litter from public right-of-ways; and Perpetual Care of 161 closed landfill sites with Technical Services 2 SWMS – Client Base Approximately 450,000 single unit homes 452,000 multi-unit homes 20,000 small commercial/institutional 6,000 litter/recycle bins / 1,000 Special Events Per Year City Agencies, Boards, Commissions and Divisions Private commercial and industrial waste accepted at Transfer Stations and Landfill 3 SWMS Assets - Facilities and Equipment Total Value of Managed Assets - $500 Million 7 Transfer Stations 6 with Household Hazardous Waste (HHW) Depots Green Lane Landfill 1 Material Recovery Facility (MRF) 1 SSO (Green Bin) Processing Facility 1 Durable Goods Reuse/Recycle Centre 4 Collection Yards and 1 Litter Collection Yard 161 Former Landfills 780 Vehicles/Heavy Equipment 4 Services and Programs Program Collection Blue Bin Recycling Bi-weekly Green Bin Weekly Waste Bi-weekly Leaf and Yard Waste Bi-weekly, (March-Dec.) -
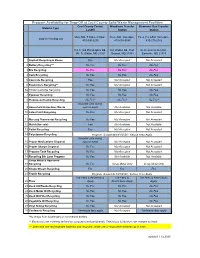
Program Availability for Drop-Off at Cecil County Solid Waste
Program Availability for Drop-Off at Cecil County Solid Waste Management Facilities Cecil County Central Woodlawn Transfer Stemmers Run Transfer Material Type Landfill Station Station Mon.-Sat. 7:30am-3:30pm, Tues.-Sat. 8am-4pm, Tues.,Fri.,&Sat. 8am-4pm, www.ccrecycling.org 410-996-6275 410-658-6646 410-275-2142 758 E. Old Philadelphia Rd., 565 Waibel Rd., Port 45 Stemmers Run Rd., (Rt. 7), Elkton, MD 21921 Deposit, MD 21904 Earleville, MD 21919 1 Asphalt Recycling & Reuse Fee Not Accepted Not Accepted 2 Battery Recycling*** No Fee No Fee No Fee 3 Bra Recycling No Fee No Fee No Fee 4 Cork Recycling No Fee No Fee No Fee 5 Concrete Recycling Fee Not Accepted Not Accepted 6 Electronics Recycling** No Fee Not Accepted Not Accepted 6a Printer Cartridge Recycling No Fee No Fee No Fee 7 Eyewear Recycling No Fee No Fee No Fee 8 Fluorescent Lamp Recycling No Fee* No Fee* No Fee* Available only during 9 Household Hazardous Waste special event Not Available Not Available 10 Latex Paint Recycling No Fee Not Accepted Not Accepted 11 Mercury Thermostat Recycling No Fee Not Accepted Not Accepted 12 Mulch (for sale) Fee Not Available Not Available 13 Pallet Recycling Fee Not Accepted Not Accepted 14 Polystyrene Recycling Program Suspended 8/1/2020 - Refuse Fees Apply Available only during 15 Proper Medications Disposal special event Not Accepted Not Accepted 16 Proper Sharps Disposal No Fee Not Accepted Not Accepted 17 Propane Tank Recycling No Fee Not Accepted Not Accepted 18 Recycling Bin Loan Program No Fee Not Available Not Available Scrap Metal -

Waste Pickers: Strategies to Enhance Livelihoods Marta Marello
Waste Pickers: Strategies to Enhance Livelihoods Marta Marello Adviser: Ann Helwege Submitted on August 23rd 2013 ACKOWLEDGMENTS I would like to thank the cooperatives in Brazil and Nicaragua who collaborated with the projects, and to Libby McDonald for welcoming me on the teams. I would also like to thank my advisor, Ann Helwege, for her help and patience during my thesis writing process; to Ethan for his love and support; to Leeann Sullivan and Matt Soergel for reviewing my work and giving helpful grammatical suggestions; and finally, to my family, who loves me and supports me in all my life choices. EXECUTIVE SUMMARY Recently in Latin America, the phenomenon of people surviving by picking through trash has received attention from artists, NGOs and policy makers. Waste pickers are poor people who sell recyclables found in trash for a living. Most are neither part of the sophisticated commercial waste management sector nor the public municipal services bureaucracy. Waste picking expanded as a profession in the mid‐twentieth century as immigrants and marginalized people could not find jobs in the formal sector and so turned to collecting and selling recyclables to make a living from the rising use of disposable goods. Despite the fact that waste picking brings numerous benefits to the environment, and to the well‐being of the community and local government, waste pickers are poor, marginalized and trapped in a cycle of low productivity. To partially solve the problem, waste pickers have organized into cooperatives, a particular business model with special attention to social needs. While this has raised income, further strategies are necessary to improve the living and working conditions of waste pickers, with positive consequences for the environment and the local community. -

Waste Cooking Oil As an Alternate Feedstock for Biodiesel Production
Energies 2008, 1, 3-18; DOI: 10.3390/en1010003 OPEN ACCESS energies ISSN 1996-1073 www.mdpi.org/energies Article Waste Cooking Oil as an Alternate Feedstock for Biodiesel Production Arjun B. Chhetri 1, K. Chris Watts 2 and M. Rafiqul Islam 1,* 1 Civil and Resources Engineering Dalhousie University, Room D510, 1360 Barrington St., Box 1000, Halifax, N.S. B3J 2X4, Canada; E-mail: [email protected] (for Arjun B. Chhetri) 2 Process Engineering, Dalhousie University, Halifax, NS, Box 1000, Halifax, N.S. B3J 2X4, Canada; Tel. (902) 494-3269; Fax 420-7639; E-mail: [email protected] * Author to whom correspondence should be addressed; E-mail: [email protected] Received: 27 March 2008 / Accepted: 9 April 2008 / Published: 10 April 2008 Abstract: As crude oil price reach a new high, the need for developing alternate fuels has become acute. Alternate fuels should be economically attractive in order to compete with currently used fossil fuels. In this work, biodiesel (ethyl ester) was prepared from waste cooking oil collected from a local restaurant in Halifax, Nova Scotia, Canada. Ethyl alcohol with sodium hydroxide as a catalyst was used for the transesterification process. The fatty acid composition of the final biodiesel esters was determined by gas chromatography. The biodiesel was characterized by its physical and fuel properties including density, viscosity, acid value, flash point, cloud point, pour point, cetane index, water and sediment content, total and free glycerin content, diglycerides and monoglycerides, phosphorus content and sulfur content according to ASTM standards. The viscosity of the biodiesel ethyl ester was found to be 5.03 mm2/sec at 40oC. -

Regional Operations and Maintenance Guide for High
Regional Operations & Maintenance Guide for High Performance Schools and Public Buildings in the Northeast and Mid-Atlantic Strategies for creating green, healthy & energy efficient existing buildings in your state or local government December 2018 About NEEP NEEP was founded in 1996 as a non-profit accelerating energy efficiency in the Northeast and Mid-Atlantic states. Today, it is one of six Regional Energy Efficiency Organizations (REEOs) funded, in part, by US Department of Energy to support state efficiency policies and programs. The High Performance Buildings Project was developed to promote operational energy savings in new and retrofitted buildings throughout the region. NEEP’s vision is that the work done today on High Performance Buildings will pave the way for the development of zero energy buildings (buildings that consume no more energy than they produce, on a broader scale throughout the region). Acknowledgments 2016 and 2018 Updates The guide was updated in 2016 and again in 2018 to reflect the most up-to-date information regarding operations and maintenance of high performance public buildings. Funding for the updates was provided, in part, by the United States Department of Energy and E4 The Future. The update was made possible by a collaborative effort from members of the Regional High Performance Schools and Public Buildings Working Group. In particular, we gratefully thank the following individuals who participated in the sub-committee to update the guide in 2016: Carolyn Dann, MA Department of Environmental Protection -

Intellectual Property Center, 28 Upper Mckinley Rd. Mckinley Hill Town Center, Fort Bonifacio, Taguig City 1634, Philippines Tel
Intellectual Property Center, 28 Upper McKinley Rd. McKinley Hill Town Center, Fort Bonifacio, Taguig City 1634, Philippines Tel. No. 238-6300 Website: http://www.ipophil.gov.ph e-mail: [email protected] Publication Date < 07 February 2020 > 1.1 ALLOWED MADRID MARKS ....................................................................................................................................... 2 Intellectual Property Center, 28 Upper McKinley Rd. McKinley Hill Town Center, Fort Bonifacio, Taguig City 1634, Philippines Tel. No. 238-6300 Website: http://www.ipophil.gov.ph e-mail: [email protected] Publication Date < 07 February 2020 > 1.1 Allowed Madrid marks International IR / Filing No. Mark Holder Nice class(es) Registration No. Date 28 October AKINDO SUSHIRO CO., LTD 1 1140881 SUSHIRO 30 and43 2019 [JP] 1; 2; 3; 4; 5; 6; 7; 8; 9; 10; 11; 12; 14; 16; 17; 18; 20; 20 May 2 1180255 ECOLAB Ecolab USA Inc. [US] 21; 22; 24; 25; 26; 2019 27; 28; 30; 35; 37; 38; 40; 41; 42; 43; 44 and45 26 3 1236181 November BULY Parfumerie Amicale [FR] 3 and4 2019 19 Rutronik Elektronische 9; 35; 38; 39; 41 4 1298027 November Rutronik Bauelemente GmbH [DE] and42 2019 14 5 1380340 November Dr.Jart+ Water Drop HAVE&BE CO., LTD. [KR] 3 2019 31 August 6 1425257 LUXIE Luxie, Inc. [US] 3 and21 2019 10 Limited Liability Company 7 1441070 September LE TARTI 30 and43 "Marka" [RU] 2018 12 8 1447107 December ENVERB Apple Inc. [US] 9 2018 28 March 9 1464527 ACTIVELY LEARN Actively Learn Inc. [US] 41 2019 Hearst Communications, Inc. 10 1470925 8 May 2019 100% CHILLED 3 and4 [US] 29 March 11 1472768 EXTAGE Co., LTD [JP] 33 2019 18 April Heidi Chocolat Suisse SA 12 1473266 5; 30 and35 2019 [CH] VALENTINO DONNA 13 1474760 1 April 2019 VALENTINO S.P.A. -

Trade Marks Journal No: 1975 , 23/11/2020 Class 34
Trade Marks Journal No: 1975 , 23/11/2020 Class 34 3918558 17/08/2018 VAIBHAV BAHARIA KOTHAR MOHALA,SHAHPURA(BHILWARA)RAJ, BHILWARA-311404 The Trade Marks Act, 1999 Address for service in India/Attorney address: VANCHINATHAN No 6, Dhanammal Street Spurtank Road Chetpet Chennai - 600031 Proposed to be Used AHMEDABAD Tobacco, smokers’ articles, matches. 3854 Trade Marks Journal No: 1975 , 23/11/2020 Class 34 3970002 10/10/2018 NIRMAL JOSHI, TRADING AS:- APNA PRODUCTS GOPALNAGAR, CHITTORGARH – 312 001 (RAJASTHAN) INDIA A Proprietorship Firm Address for service in India/Attorney address: TRADESAFE GHANSHYAM HOUSE, BUNGLOW NO. 9, SHRINAGAR SOCIETY, OPP. SARDAR PATEL STADIUM, NEAR GOLDEN TRIANGLE, AHMEDABAD – 380 014 (GUJARAT) INDIA Used Since :27/08/1984 AHMEDABAD TOBACCO, UNMANUFACTURED TOBACCO, READY TO EAT FILTER TOBACCO, LIME MIX CHEWING TOBACCO, ZARDA. .. THIS IS SUBJECT TO ASSOCIATION WITH REGISTERED/PENDING REGISTRATION NO..3970003, 3970004, 1284067.. 3855 Trade Marks Journal No: 1975 , 23/11/2020 Class 34 4001997 19/11/2018 DS INNOVATIVE PRODUCTS LLP 4828/24, plot no. 2 G/F Basement, Ward no. XI, Daryaganj, Delhi 110002 A COMPANY INCORPORATED UNDER THE INDIAN COMPANIES ACT Address for service in India/Agents address: THE ACME COMPANY B-41, NIZAMUDDIN EAST, NEW DELHI - 110013. Proposed to be Used DELHI Tobacco; smokers’ articles; matches, lighters for smokers 3856 Trade Marks Journal No: 1975 , 23/11/2020 Class 34 4001999 19/11/2018 DS INNOVATIVE PRODUCTS LLP 4828/24, plot no. 2 G/F Basement, Ward no. XI, Daryaganj, Delhi 110002 A COMPANY INCORPORATED UNDER THE INDIAN COMPANIES ACT Address for service in India/Agents address: THE ACME COMPANY B-41, NIZAMUDDIN EAST, NEW DELHI - 110013. -

Norsk Varemerketidende Nr 41/18
. nr 41/18 - 2018.10.08 NO årgang 108 ISSN 1503-4925 Norsk varemerketidende er en publikasjon som inneholder kunngjøringer innenfor varemerkeområdet BESØKSADRESSE Sandakerveien 64 POSTADRESSE Postboks 4863 Nydalen 0422 Oslo E-POST [email protected] TELEFON +47 22 38 73 00 8.00-15.45 innholdsfortegnelse og inid-koder 2018.10.08 - 41/18 Innholdsfortegnelse: Registrerte varemerker ......................................................................................................................................... 3 Internasjonale varemerkeregistreringer ............................................................................................................ 37 Ansvarsmerker .................................................................................................................................................... 91 Innsigelser ............................................................................................................................................................ 92 Avgjørelse fra Klagenemnda .............................................................................................................................. 93 Merkeendringer .................................................................................................................................................... 94 Begrensing i varefortegnelsen for internasjonale varemerkeregistreringer ................................................. 95 Trekninger/slettelser begjært av søker/innehaver ........................................................................................ -

EPD Response to Bob Palmer January 18, 2012 EPAC General Background Questions
EPD Response to Bob Palmer January 18, 2012 EPAC General Background Questions General Questions 1. Please provide a list of major EPD accomplishments in recent years. Pollution Prevention Major Accomplishments of Pollution Prevention Division over last 5 years. Provided local technical oversight of Cabot-Koppers Superfund Site clean-up plans and investigation activities on behalf of Alachua County and the City of Gainesville, resulting in increased attention by USEPA to offsite soil and dust contamination issues and improved remedial technologies for increased protection for neighboring residents and groundwater resources Thirty-two (32) petroleum contaminated sites in Alachua Countyhave been cleaned-up including major petroleum cleanup at the Poole Roofing/ Depot Park site in downtown Gainesville,. Active remediation systems have been installed on Hogtown Creek to clean-up gasoline discharge into the creek. Provided annual regulatory oversight resulting in upgrade of 350 underground fuel storage tanks in Alachua County to double wall tanks which provided increased protection of drinking water resources from petroleum fuel leaks. Established a first-in-the-state unwanted pharmaceuticals/medicines collection and disposal program in Alachua County with multiple drop-off locations to protect water resources from contamination. Established a waste vegetable oil recycling and biodiesel fuel production capability at the Hazardous Waste Collection Center (HWCC). Established capability to operate the HWCC facility off-the grid with 100% biodiesel powered electrical generator. Installed solar photovoltaic electrical generation at the HWCC to offset facility electrical usage and generate revenues for the County through the GRU FIT program. Established and expanded a model electronic scrap recycling and reuse capability at the Hazardous Waste Collection Center. -

Waste Vegetable Oil Recycling for Bio-Diesel Production in Essex & Cambridgeshire
Waste Vegetable Oil Recycling for Bio-diesel Production in Essex & Cambridgeshire WasteWISE Overview Report 2 MAY 2003 PLEASE SEND FURTHER COMMENTS TO: Andrew Stevens Working in Social Enterprise Project, Anglia Polytechnic University Ashby House, Bishop Hall Lane, Chelmsford CM1 1SQ email [email protected] or [email protected] tel: 01245 493131 x4967/4972 fax: 01245 493235 website: www.wastewise.net CONTENTS Executive Summary 1 Background 2 Estimated Quantities Available for Recycling 3 Financial Overview 4 Social Employment Opportunities 5 Environmental Issues 6 Partner Organisations 7 Key Issues for Further Analysis/Discussion 8 Next Steps/Detailed Financial Analysis 9 Contacts/Information Sources Appendix 1 Bio-diesel Technical Information Appendix 2 Estimated WasteWISE Bio-diesel Production Costs 1 Executive Summary • It is estimated that 75,000 tonnes of waste vegetable oil arise from catering and industrial sources annually in the UK. Estimates for Essex and Cambridge are 1,850 tonnes and 750 tonnes respectively. In addition, up to 3 times this amount of waste oil may be available from households. • Only a small fraction of this is currently recycled. Current use to manufacture animal feed will soon be prohibited within the EU as a result of BSE transmission control. • Vegetable oil can be efficiently converted to bio-diesel using a simple process. The estimated annual quantities of waste oil produced in Essex and Cambridge could produce around 2.4 million litres of fuel. • Bio-diesel provides a cleaner burning, more environmentally friendly substitute to ordinary diesel. No vehicle modifications are necessary. • A WasteWISE scheme is proposed in which bio-diesel is manufactured from waste vegetable oil at a VAT inclusive cost of around 50p per litre. -
BU Zero Waste Plan
1.5M HOT-DRINK CUPS PER YEAR IS JUST THE BEGINNING Boston University Zero Waste Plan Reimagining Waste as a Resource Boston University Sustainability Boston University Sustainability Sustainability Table of Contents Executive Summary 2 Background 5 Existing System 8 Challenges and Opportunities 11 ZERO WASTE INITIATIVES 12 Redesign 12 Reduce 12 Reuse 13 Recycle/Compost 14 Culture Change 14 Market Development 15 Impacts 16 Costs and Staffing 18 Timeline 19 Appendix A – Zero Waste Implementation Task Force 20 Appendix B – Stakeholder and Community Engagement 23 Appendix C – Diversion Analysis 24 Appendix D – TRUE Zero Waste Analysis 25 1 Executive Summary ZERO WASTE, allows for greater efficiency at the institutional A PARADIGM SHIFT level. Improved diversion rates result from this whole systems approach. For decades, it has been widely demon- Just like “zero accidents on the job site” and strated that the materials we make and “zero defects in production,” Zero Waste is a goal use, and how we dispose of them, have that is embraced by sustainable communities, significant environmental impacts. These businesses, and institutions. impacts range from growing landfills to Businesses, including Toyota and Sierra Nevada air pollution and water pollution, all of Brewing, are exemplars of Zero Waste practices. which have impacts on human health. With the international market for recyclables facing In the 1980s, “waste-to-energy” inciner- upheaval, especially from China’s National Sword ation seemed to be the solution, with a ban on contaminated recycling streams, many 75% reduction of volume to landfill with municipalities are also developing Zero Waste plans, only the residues from combustion need- including the City of Boston. -

Green Energy Options for Consumer-Owned Business DE-AC36-99-GO10337
A national laboratory of the U.S. Department of Energy Office of Energy Efficiency & Renewable Energy National Renewable Energy Laboratory Innovation for Our Energy Future Green Energy Options for Subcontract Report NREL/SR-560-39465 Consumer-Owned Business May 2006 Co-opPlus of Western Massachusetts Greenfield, Massachusetts NREL is operated by Midwest Research Institute ● Battelle Contract No. DE-AC36-99-GO10337 Green Energy Options for Subcontract Report NREL/SR-560-39465 Consumer-Owned Business May 2006 Co-opPlus of Western Massachusetts Greenfield, Massachusetts NREL Technical Monitor: Holly Thomas Prepared under Subcontract No. ZAT-4-33658-01 National Renewable Energy Laboratory 1617 Cole Boulevard, Golden, Colorado 80401-3393 303-275-3000 • www.nrel.gov Operated for the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy by Midwest Research Institute • Battelle Contract No. DE-AC36-99-GO10337 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof.