Ocular Inflammation Associated with Systemic Infection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
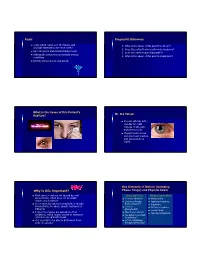
Why Is This Important? Phone Triage) and Physical Exam
Goals Diagnostic Dilemmas Learn which features of the history and 1. What is the cause of this patient’s red eye? physical examination are most useful 2. Does this patient have a pathologic headache? Use risk scores and clinical decision tools 3. Does this patient have endocarditis? Distinguish benign from potentially serious 4. What is the cause of this patient’s back pain? conditions Identify clinical pearls and pitfalls What is the Cause of this Patient’s Mr. Ira Tatedi Red Eye? 32 year old man with one day h/o mild redness of OD, pain, and photophobia. Physical exam shows circumcorneal injection, and visual acuity is 20/80. Key Elements of History (including Why is this Important? Phone Triage) and Physical Exam Most cases of red eye are caused by viral History and Triage Physical Examination conjunctivitis, which does not generally Is vision affected? Visual acuity require any treatment Is there a foreign Pupil size/reactivity Some cases are caused by bacterial or allergic body sensation? Discharge conjunctivitis, for which specific treatment is Is there Pattern of redness indicated photophobia? Foreign body A minority of cases are caused by other Was there trauma? Hypopyon/hyphema conditions, which require urgent or emergent Are patient a contact referral to an ophthalmologist lens wearer? It is essential to be able to distinguish them Is there discharge from one another throughout the day? Anatomy/Differential Diagnosis Conjunctivitis Viral Conjunctivitis – Erythema with co-existing URI – Watery, serous discharge -

Cytomegalovirus Retinitis: a Manifestation of the Acquired Immune Deficiency Syndrome (AIDS)*
Br J Ophthalmol: first published as 10.1136/bjo.67.6.372 on 1 June 1983. Downloaded from British Journal ofOphthalmology, 1983, 67, 372-380 Cytomegalovirus retinitis: a manifestation of the acquired immune deficiency syndrome (AIDS)* ALAN H. FRIEDMAN,' JUAN ORELLANA,'2 WILLIAM R. FREEMAN,3 MAURICE H. LUNTZ,2 MICHAEL B. STARR,3 MICHAEL L. TAPPER,4 ILYA SPIGLAND,s HEIDRUN ROTTERDAM,' RICARDO MESA TEJADA,8 SUSAN BRAUNHUT,8 DONNA MILDVAN,6 AND USHA MATHUR6 From the 2Departments ofOphthalmology and 6Medicine (Infectious Disease), Beth Israel Medical Center; 3Ophthalmology, "Medicine (Infectious Disease), and 'Pathology, Lenox Hill Hospital; 'Ophthalmology, Mount Sinai School ofMedicine; 'Division of Virology, Montefiore Hospital and Medical Center; and the 8Institute for Cancer Research, Columbia University College ofPhysicians and Surgeons, New York, USA SUMMARY Two homosexual males with the 'gay bowel syndrome' experienced an acute unilateral loss of vision. Both patients had white intraretinal lesions, which became confluent. One of the cases had a depressed cell-mediated immunity; both patients ultimately died after a prolonged illness. In one patient cytomegalovirus was cultured from a vitreous biopsy. Autopsy revealed disseminated cytomegalovirus in both patients. Widespread retinal necrosis was evident, with typical nuclear and cytoplasmic inclusions of cytomegalovirus. Electron microscopy showed herpes virus, while immunoperoxidase techniques showed cytomegalovirus. The altered cell-mediated response present in homosexual patients may be responsible for the clinical syndromes of Kaposi's sarcoma and opportunistic infection by Pneumocystis carinii, herpes simplex, or cytomegalovirus. http://bjo.bmj.com/ Retinal involvement in adult cytomegalic inclusion manifestations of the syndrome include the 'gay disease (CID) is usually associated with the con- bowel syndrome9 and Kaposi's sarcoma. -

Differentiate Red Eye Disorders
Introduction DIFFERENTIATE RED EYE DISORDERS • Needs immediate treatment • Needs treatment within a few days • Does not require treatment Introduction SUBJECTIVE EYE COMPLAINTS • Decreased vision • Pain • Redness Characterize the complaint through history and exam. Introduction TYPES OF RED EYE DISORDERS • Mechanical trauma • Chemical trauma • Inflammation/infection Introduction ETIOLOGIES OF RED EYE 1. Chemical injury 2. Angle-closure glaucoma 3. Ocular foreign body 4. Corneal abrasion 5. Uveitis 6. Conjunctivitis 7. Ocular surface disease 8. Subconjunctival hemorrhage Evaluation RED EYE: POSSIBLE CAUSES • Trauma • Chemicals • Infection • Allergy • Systemic conditions Evaluation RED EYE: CAUSE AND EFFECT Symptom Cause Itching Allergy Burning Lid disorders, dry eye Foreign body sensation Foreign body, corneal abrasion Localized lid tenderness Hordeolum, chalazion Evaluation RED EYE: CAUSE AND EFFECT (Continued) Symptom Cause Deep, intense pain Corneal abrasions, scleritis, iritis, acute glaucoma, sinusitis, etc. Photophobia Corneal abrasions, iritis, acute glaucoma Halo vision Corneal edema (acute glaucoma, uveitis) Evaluation Equipment needed to evaluate red eye Evaluation Refer red eye with vision loss to ophthalmologist for evaluation Evaluation RED EYE DISORDERS: AN ANATOMIC APPROACH • Face • Adnexa – Orbital area – Lids – Ocular movements • Globe – Conjunctiva, sclera – Anterior chamber (using slit lamp if possible) – Intraocular pressure Disorders of the Ocular Adnexa Disorders of the Ocular Adnexa Hordeolum Disorders of the Ocular -

CAUSES, COMPLICATIONS &TREATMENT of A“RED EYE”
CAUSES, COMPLICATIONS & TREATMENT of a “RED EYE” 8 Most cases of “red eye” seen in general practice are likely to be conjunctivitis or a superficial corneal injury, however, red eye can also indicate a serious eye condition such as acute angle glaucoma, iritis, keratitis or scleritis. Features such as significant pain, photophobia, reduced visual acuity and a unilateral presentation are “red flags” that a sight-threatening condition may be present. In the absence of specialised eye examination equipment, such as a slit lamp, General Practitioners must rely on identifying these key features to know which patients require referral to an Ophthalmologist for further assessment. Is it conjunctivitis or is it something more Iritis is also known as anterior uveitis; posterior uveitis is serious? inflammation of the choroid (choroiditis). Complications include glaucoma, cataract and macular oedema. The most likely cause of a red eye in patients who present to 4. Scleritis is inflammation of the sclera. This is a very rare general practice is conjunctivitis. However, red eye can also be presentation, usually associated with autoimmune a feature of a more serious eye condition, in which a delay in disease, e.g. rheumatoid arthritis. treatment due to a missed diagnosis can result in permanent 5. Penetrating eye injury or embedded foreign body; red visual loss. In addition, the inappropriate use of antibacterial eye is not always a feature topical eye preparations contributes to antimicrobial 6. Acid or alkali burn to the eye resistance. The patient history will usually identify a penetrating eye injury Most general practice clinics will not have access to specialised or chemical burn to the eye, but further assessment may be equipment for eye examination, e.g. -

Herpetic Viral Retinitis
American Journal of Virology 2 (1): 25-35, 2013 ISSN: 1949-0097 ©2013 Science Publication doi:10.3844/ajvsp.2013.25.35 Published Online 2 (1) 2013 (http://www.thescipub.com/ajv.toc) Herpetic Viral Retinitis Hidetaka Noma Department of Ophthalmology, Yachiyo Medical Center, Tokyo Women’s Medical University, Chiba, Japan Received 2012-05-30, Revised 2012-07-09; Accepted 2013-07-22 ABSTRACT Human Herpes Virus (HHV) is a DNA virus and is the most important viral pathogen causing intraocular inflammation. HHV is classified into types 1-8. Among these types, HHV-1, HHV-2, HHV-3 Varicella Zoster Virus (VZV) and HHV-5 Cytomegalovirus (CMV) are known to cause herpetic viral retinitis, including acute retinal necrosis and CMV retinitis. Herpes viral retinitis can be diagnosed from characteristic ocular findings and viral identification by PCR of the aqueous humor. Recently, therapy has become more effective than in the past. Herpes viral retinitis gradually progresses if appropriate treatment is not provided with regard to the patient’s immune status. Further advances in diagnostic methods and treatment are required in the future. Keywords: Human Herpes Virus (HHV), Varicella Zoster Virus (VZV), Acute Retinal Necrosis, Polymerase Chain Reaction (PCR), CMV Retinitis, Pathogen Causing Intraocular Inflammation 1. INTRODUCTION by Young and Bird (1978). In the 1980s, the etiology of the disease was shown to be infection by HSV and Human Herpes Virus (HHV) is a DNA virus and VSV. Widespread adoption of the Polymerase Chain the most important viral pathogen causing intraocular Reaction (PCR) method from early the 1990 made it inflammation. It is classified into types 1-8, among possible to easily detect the presence of intraocular which HHV-1, HHV-2, HHV-3 (Varicella Zoster viruses, after which many cases of ARN were Virus: VZV) and HHV-5 Cytomegalovirus (CMV) are diagnosed. -

Herpetic Corneal Infections
FocalPoints Clinical Modules for Ophthalmologists VOLUME XXVI, NUMBER 8 SEPTEMBER 2008 (MODULE 2 OF 3) Herpetic Corneal Infections Sonal S. Tuli, MD Reviewers and Contributing Editors Consultants George A. Stern, MD, Editor for Cornea & External Disease James Chodosh, MD, MPH Kristin M. Hammersmith, MD, Basic and Clinical Science Course Faculty, Section 8 Kirk R. Wilhelmus, MD, PhD Christie Morse, MD, Practicing Ophthalmologists Advisory Committee for Education Focal Points Editorial Review Board George A. Stern, MD, Missoula, MT Claiming CME Credit Editor in Chief, Cornea & External Disease Thomas L. Beardsley, MD, Asheville, NC Academy members: To claim Focal Points CME cred- Cataract its, visit the Academy web site and access CME Central (http://www.aao.org/education/cme) to view and print William S. Clifford, MD, Garden City, KS Glaucoma Surgery; Liaison for Practicing Ophthalmologists Advisory your Academy transcript and report CME credit you Committee for Education have earned. You can claim up to two AMA PRA Cate- gory 1 Credits™ per module. This will give you a maxi- Bradley S. Foster, MD, Springfield, MA Retina & Vitreous mum of 24 credits for the 2008 subscription year. CME credit may be claimed for up to three (3) years from Anil D. Patel, MD, Oklahoma City, OK date of issue. Non-Academy members: For assistance Neuro-Ophthalmology please send an e-mail to [email protected] or a Eric P. Purdy, MD, Fort Wayne, IN fax to (415) 561-8575. Oculoplastic, Lacrimal, & Orbital Surgery Steven I. Rosenfeld, MD, FACS, Delray Beach, FL Refractive Surgery, Optics & Refraction C. Gail Summers, MD, Minneapolis, MN Focal Points (ISSN 0891-8260) is published quarterly by the American Academy of Ophthalmology at 655 Beach St., San Francisco, CA 94109-1336. -
UVEITIS Eye74 (1)
UVEITIS Eye74 (1) Uveitis Last updated: May 9, 2019 Classification .................................................................................................................................... 1 Etiologic categories .......................................................................................................................... 2 Treatment ......................................................................................................................................... 2 Complications ................................................................................................................................... 2 COMMON UVEITIC SYNDROMES ............................................................................................................. 2 Masquerade Syndromes ................................................................................................................... 3 UVEITIS - heterogenous ocular diseases - inflammation of any component of uveal tract (iris, ciliary body, choroid). CLASSIFICATION ANTERIOR UVEITIS (most common uveitis) - localized to anterior segment - iritis and iridocyclitis. IRITIS - white cells confined solely to anterior chamber. IRIDOCYCLITIS - cellular activity also involves retrolental vitreous. etiology (most do not have underlying systemic disease): 1) idiopathic postviral syndrome (most commonly 38-60%) 2) HLA-B27 syndromes, many arthritic syndromes (≈ 17%) 3) trauma (5.7%) 4) herpes simplex, herpes zoster disease (1.9-12.4%) 5) iatrogenic (postoperative). tends to -

PEA's NEWSLETTER July Welcome to PEA's Very First Newsletter, Prisms
Issue 1 | Volume 1 | July 2019 Pacific Eye Associates www.pacificeye.com Prisms415.923.3007 PEA'S NEWSLETTER July Welcome to PEA's very first newsletter, Prisms. In our quarterly newsletter we'll deliver updates about our office, doctors, and eye treatments, as well as create a community of like- minded professionals. We'll keep each of our newsletters to a five minute read. Time is precious and so are your eyes, no dry eyes on our account! We count on doctors to make our newsletters better each time. Please email us with topics about which you would like to read. In this quarter's newsletter: - Dr. Oxford writes about our newest dry eye treatment, LipiFlow. - Dr. Heiden awarded the Outstanding Humanitarian Service Award. Dr. David Heiden received the 2018 Outstanding Humanitarian Service Award from the American Academy of Ophthalmology. He was nominated by Pacific Vision Foundation and Seva Foundation. Out of 32,000 members, only two physicians were selected for this honor. Dr. Heiden received the award in recogni- tion of his work to bring state- of- the- art blindness prevention techniques to HIV/ AIDS patients in politically unstable and poverty- stricken environments across the globe. He pioneered the practice of training primary care AIDS doctors how to use eye exams to diagnose and treat Cytomegalovirus (CM V) retinitis, a disease that can increase AIDS- related mortality and lead to sudden, irreversible blindness. He also trained AIDS doctors how to perform intraocular injection of medication to treat CM V retinitis, something that had never been considered before. CM V retinitis is a complication of AIDS and now almost forgotten in the David Heiden, M .D. -

Pediatric Pharmacology and Pathology
7/31/2017 In the next 2 hours……. Pediatric Pharmacology and Pathology . Ocular Medications and Children The content of th is COPE Accredited CE activity was prepared independently by Valerie M. Kattouf O.D. without input from members of the optometric community . Brief review of examination techniques/modifications for children The content and format of this course is presented without commercial bias and does not claim superiority of any commercial product or service . Common Presentations of Pediatric Pathology Valerie M. Kattouf O.D., F.A.A.O. Illinois College of Optometry Chief, Pediatric Binocular Vision Service Associate Professor Ocular Medications & Children Ocular Medications & Children . Pediatric systems differ in: . The rules: – drug excretion – birth 2 years old = 1/2 dose kidney is the main site of drug excretion – 2-3 years old = 2/3 dose diminished 2° renal immaturity – > 3 years old = adult dose – biotransformation liver is organ for drug metabolism Impaired 2° enzyme immaturity . If only 50 % is absorbed may be 10x maximum dosage Punctal Occlusion for 3-4 minutes ↓ systemic absorption by 40% Ocular Medications & Children Ocular Medications & Children . Systemic absorption occurs through….. Ocular Meds with strongest potential for pediatric SE : – Mucous membrane of Nasolacrimal Duct 80% of each gtt passing through NLD system is available for rapid systemic absorption by the nasal mucosa – 10 % Phenylephrine – Conjunctiva – Oropharynx – 2 % Epinephrine – Digestive system (if swallowed) Modified by variation in Gastric pH, delayed gastric emptying & intestinal mobility – 1 % Atropine – Skin (2° overflow from conjunctival sac) Greatest in infants – 2 % Cyclopentalate Blood volume of neonate 1/20 adult Therefore absorbed meds are more concentrated at this age – 1 % Prednisone 1 7/31/2017 Ocular Medications & Children Ocular Medications & Children . -

Retinitis Pigmentosa, Ataxia, and Peripheral Neuropathy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.46.3.206 on 1 March 1983. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1983;46:206-213 Retinitis pigmentosa, ataxia, and peripheral neuropathy RR TUCK, JG McLEOD From the Department ofMedicine, University ofSydney, Australia SUMMARY The clinical features of four patients with retinitis pigmentosa, ataxia and peripheral neuropathy but with no increase in serum phytanic acid are reported. Three patients also had sensorineural deafness and radiological evidence of cerebellar atrophy. Nerve conduction studies revealed abnormalities of sensory conduction and normal or only mild slowing of motor conduc- tion velocity. Sural nerve biopsy demonstrated a reduction in the density of myelinated fibres. There were no onion bulb formations. These cases clinically resemble Refsum's disease, but differ in having no detectable biochemical abnormality, and a peripheral neuropathy which is not hypertrophic in type. They may represent unusual cases of spinocerebellar degeneration. Retinitis pigmentosa occurs infrequently as an iso- (WAIS). He had a speech impediment but was not dysar- Protected by copyright. lated finding in otherwise healthy individuals and thric. He was of short stature, had a small head and pes families. Its association with deafness, with or with- cavus but no kyphoscoliosis. His visual acuity in the right eye was 6/60 while in the left he could count fingers only. out other neurological abnormalities is much less The right visual field was constricted but the left could not common but nevertheless well recognised.1 In be tested. The optic discs were pale, the retinal vessels heredopathia atactica polyneuritiformis (Refsum's small in diameter and throughout the retinae there was disease), abetalipoproteinaemia, and the Keams- scattered "bone corpuscle" pigmentation. -

View Returned the Following Day, Less Than OR
s s CONSEQUENTIAL CASES CASES THAT CHANGED THE WAY WE PRACTICE Three retina specialists share stories of patient encounters that left lasting impressions. LET THERE BE LIGHT By Ninel Z. Gregori, MD vision requires daily practice and hard my career in the direction of other A 52-year-old patient work. The patient had an intense innovative surgeries and clinical trials who had seen nothing rehabilitation process ahead of her at Bascom Palmer. but weak light for to help her to make sense of the And it did much more: It helped me 19 years since devel- lights. She is now able to identify light truly understand and appreciate the oping end-stage retinitis pigmentosa objects against dark backgrounds, value of careful preoperative counsel- (RP) came into my care. Then, in 2013, sort light and dark clothes, see light ing and setting realistic expectations the long-awaited promise of artificial coming in the windows, identify the for patients undergoing new treat- vision became a reality because the handle on the refrigerator, and even ments. After observing the experiences US FDA had approved the first retinal identify some letters, numbers, and of my patient receiving an Argus II prosthesis for patients with RP. I dis- simple words on a computer screen. implant, I now make sure to carefully cussed the Argus II Retinal Prosthesis She cannot, however, recognize explain the risks and limitations of System (Second Sight) with the objects and people in the environ- artificial vision options to patients who patient, and we agreed that she was a ment consistently, see faces, or read. -

Upper Eyelid Ptosis Revisited Padmaja Sudhakar, MBBS, DNB (Ophthalmology) Qui Vu, BS, M3 Omofolasade Kosoko-Lasaki, MD, MSPH, MBA Millicent Palmer, MD
® AmericAn JournAl of clinicAl medicine • Summer 2009 • Volume Six, number Three 5 Upper Eyelid Ptosis Revisited Padmaja Sudhakar, MBBS, DNB (Ophthalmology) Qui Vu, BS, M3 Omofolasade Kosoko-Lasaki, MD, MSPH, MBA Millicent Palmer, MD Abstract Epidemiology of Ptosis Blepharoptosis, commonly referred to as ptosis is an abnormal Although ptosis is commonly encountered in patients of all drooping of the upper eyelid. This condition has multiple eti- ages, there are insufficient statistics regarding the prevalence ologies and is seen in all age groups. Ptosis results from a con- and incidence of ptosis in the United States and globally.2 genital or acquired weakness of the levator palpebrae superioris There is no known ethnic or sexual predilection.2 However, and the Muller’s muscle responsible for raising the eyelid, dam- there have been few isolated studies on the epidemiology of age to the nerves which control those muscles, or laxity of the ptosis. A study conducted by Baiyeroju et al, in a school and a skin of the upper eyelids. Ptosis may be found isolated, or may private clinic in Nigeria, examined 25 cases of blepharoptosis signal the presence of a more serious underlying neurological and found during a five-year period that 52% of patients were disorder. Treatment depends on the underlying etiology. This less than 16 years of age, while only 8% were over 50 years review attempts to give an overview of ptosis for the primary of age. There was a 1:1 male to female ratio in the study with healthcare provider with particular emphasis on the classifica- the majority (68%) having only one eye affected.