Research Review 2001
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

D-PHYS Master of Science – ETH Zurich and Ecole Polytechnique Paris Specialization in High Energy Physics
D-PHYS Master of Science – ETH Zurich and Ecole Polytechnique Paris Specialization in High Energy Physics Study Guide Department of Physics Content Introduction 4 1 Master program 5 2 Performance Assessment 10 3 Program requirements, application and admission 18 4 Useful information about ETH Zurich and EP Paris 21 5 Appendix 26 Imprint Editorial staff Günther Dissertori and Matthias Gaberdiel Photograph Heidi Hostettler Graphic Design Amanda Eisenhut 3 Introduction 1 Master program ETH Zurich and Ecole Polytechnique (EP) Paris offer a Joint Master The aim of this Master specialization is to 1.1 Tutor System offer a coherent theoretical and experi program with specialization in High Energy Physics (HEP). High Energy Each student in the Master program in mental education in High Energy Physics, Physics studies the elementary constituents of matter and the associated High Energy Physics will be allocated a covering a wide spectrum of areas and ap fundamental forces. The tools for these studies are experiments at tutor through the academic board. The plications: particle physics, astroparticle tutor gives academic advice and helps with particle accelerators operating at very high energies or at very high physics, the Standard Model of the elec the coordination of the program. In parti beam intensities, as well as ultra-sensitive large-mass detectors. These troweak interactions and its supersym cular, the tutor advises the student in the experimental setups give sensitivity to the laws of physics at very short metric extensions, strong interactions and choice of courses for the second year, given distances. The Large Hadron Collider (LHC), launched in September 2008 quantum chromodynamics, nuclear phys the selection of courses taken in the first ics, general relativity and quantum gravity at CERN, is the most spectacular realization of such a tool to date. -

Differentiation of Physical and Chemical Cross-Linking in Gelatin Methacryloyl Hydrogels Lisa Rebers 1,+ , Raffael Reichsöllner 2,+ , Sophia Regett 1, Günter E
Supplementary Information Differentiation of Physical and Chemical Cross-Linking in Gelatin Methacryloyl Hydrogels Lisa Rebers 1,+ , Raffael Reichsöllner 2,+ , Sophia Regett 1, Günter E. M. Tovar 1,3, *, Kirsten Borchers 1,3 , Stefan Baudis 2 and Alexander Southan 1, * 1 Institute of Interfacial Process Engineering and Plasma Technology, University of Stuttgart, Stuttgart, Germany. 2 Christian Doppler Laboratory for Advanced Polymers for Biomaterials and 3D Printing, Institute of Applied Synthetic Chemistry, TU Wien, Vienna, Austria. 3 Fraunhofer Institute for Interfacial Engineering and Biotechnology, Stuttgart, Germany. * [email protected], [email protected] + These authors contributed equally. Figure S1: 1H-NMR spectra of gelatin used for methacryloylation (GM) (and acetylation (GMA)) and its derivatives. Unmodified lysine groups, only present in the spectrum of the unmodified gelatin, were highlighted in dark grey, acrylic protons of methacryloyl groups in light grey. The figure was created with Origin 2019b (https://www.originlab.com/2019b). Figure S2: Utilized temperature profile for physical gelation prior to chemical cross-linking. The 37 °C warm GM-solutions were cooled for 20 min to 21 °C followed by cooling to 4 °C 40 min (green dotted lines). Afterwards, infrared spectroscopy (IR) acquisition was started (orange dotted line) and the UV light was turned on 5 s later (blue dotted line). The figure was created with Origin 2019b (https://www.originlab.com/2019b). Figure S3: Circular dichroism (CD) spectra of gelatin ( A), GM2 ( B), GM2A8 ( C) and GM10 ( D). CD spectra were recorded at 37 °C or after cooling procedure (37 °C to 21 °C for 20 min followed by cooling to 4 °C for 40 min). -

Probabilistic Engineering Mechanics
PROBABILISTIC ENGINEERING MECHANICS AUTHOR INFORMATION PACK TABLE OF CONTENTS XXX . • Description p.1 • Audience p.2 • Impact Factor p.2 • Abstracting and Indexing p.2 • Editorial Board p.2 • Guide for Authors p.4 ISSN: 0266-8920 DESCRIPTION . This journal provides a forum for scholarly work dealing primarily with probabilistic and statistical approaches to contemporary solid/structural and fluid mechanics problems encountered in diverse technical disciplines such as aerospace, civil, marine, mechanical, and nuclear engineering. The journal aims to maintain a healthy balance between general solution techniques and problem- specific results, encouraging a fruitful exchange of ideas among disparate engineering specialities. From time to time, review papers will be published to provide research and development oriented readers with state-of-the-art analyses of various areas of current interest. In addition, occasional papers of tutorial nature help enhance practice oriented readers' knowledge of the basic probabilistic and statistical techniques that are essential in present-day engineering practice design. In consultation with the editors, distinguished members of the probabilistic mechanics community may serve as guest editors for a special issue dedicated to a particular theme. Fields Covered: Aerospace engineering: • Damage-tolerant and durability design of aircraft • Load spectra characterisation • Random vibration of aerospace structures Civil engineering: • Geotechnical applications • Natural hazards such as earthquakes, hurricanes, -

Research, Art and Impact Assessment
Research, Art and Impact Assessment Aalto University Aalto University Research, Art and Impact Assessment RAI 2018 Report Ella Bingham, Krisztina Cziner, Marjo Kettunen and Tuija Pulkkinen (ed.) Publisher: Aalto University Layout: Matti Ahlgren and Päivi Kekäläinen Copyediting: Heidi Henrickson Cover photo and photos on pages 4, 22, 178: Unto Rautio/Aalto University Print: Unigrafia 2019 Available online at https://www.aalto.fi/research-art/research-assessments ISBN 978-952-60-3762-2 1 2 Contents President's greetings 5 RAI 2018 – Why? 7 Executive Summary 8 Aalto University's Mission and Strategic Development Actions 11 Organization and Implementation of the Assessment 15 Assessment Fields and Units of Assessment 18 Assessment Panels, Report and Criteria 19 Utilisation of the Assessment Results 21 Main findings and recommendations 23 Field 1: Arts, Design and Architecture 30 Field 2: Business and Economics 48 Field 3a: Chemical engineering and physics 66 Field 3b: Engineering 82 Field 4: ICT and Mathematics 96 Field 5: Energy 114 Field 6: Health and Wellbeing 130 Field 7: Living environments 144 Field 8: Innovation Ecosystem 156 Elements of Assessment 180 Panels 182 Assessment organisation 184 3 4 President's greetings Nearly 10 years since founding – 42 international specialists assessed the development of Aalto University In 2010, Helsinki University of Technology, the University of Art and Design, and the Helsinki School of Economics merged to form Aalto University, which was given a special national task: to strengthen the innovative capacity of Finland through first-class research, artistic activities, and education. The aim was to create a new kind of research university that combines high societal relevance with uncompromising scientific rigor and groundbreaking art. -

WUDR Biology
www.cicerobook.com Biology 2021 TOP-500 Double RankPro 2021 represents universities in groups according to the average value of their ranks in the TOP 500 of university rankings published in a 2020 World University Country Number of universities Rank by countries 1-10 California Institute of Technology Caltech USA 1-10 Harvard University USA Australia 16 1-10 Imperial College London United Kingdom Austria 2 1-10 Massachusetts Institute of Technology USA Belgium 7 1-10 Stanford University USA Brazil 1 1-10 University College London United Kingdom Canada 12 1-10 University of California, Berkeley USA China 14 1-10 University of Cambridge United Kingdom Czech Republic 1 1-10 University of Oxford United Kingdom Denmark 4 1-10 Yale University USA Estonia 1 11-20 Columbia University USA Finland 4 11-20 Cornell University USA France 9 11-20 ETH Zürich-Swiss Federal Institute of Technology Zurich Switzerland Germany 26 11-20 Johns Hopkins University USA Greece 1 11-20 Princeton University USA Hong Kong 3 11-20 University of California, Los Angeles USA Ireland 4 11-20 University of California, San Diego USA Israel 4 11-20 University of Pennsylvania USA Italy 11 11-20 University of Toronto Canada Japan 6 11-20 University of Washington USA Netherlands 9 21-30 Duke University USA New Zealand 2 21-30 Karolinska Institutet Sweden Norway 3 21-30 Kyoto University Japan Portugal 2 21-30 Ludwig-Maximilians University of Munich Germany Rep.Korea 5 21-30 National University of Singapore Singapore Saudi Arabia 2 21-30 New York University USA Singapore 2 21-30 -

Entwicklungsplan 2025 Der Technischen Universität Wien DOKUMENTENHISTORIE
Entwicklungsplan 2025 der Technischen Universität Wien DOKUMENTENHISTORIE Version Datum Änderungen 1.0 Dezember 2017 2.0 April 2018 Tabelle 2: geplante Professuren 2018-2021 3.0 Mai 2019 Tabelle 2: geplante Professuren 2018-2021 Entwicklungsplan 2025 Wien, Dezember 2017 Entwicklungsplan der TU Wien 2025 3 von 52 HERZLICHEN DANK! Dieses Strategiepapier ist als Resultat eines durch das Rektorat der TU Wien initiierten Dialog- prozesses unter engagierter Mitwirkung von Vertreter_innen aller Personengruppen der TU Wien entstanden. Unser besonderer Dank gilt allen involvierten Personen, die einen Beitrag dazu geleistet haben. Entwicklungsplan der TU Wien 2025 4 von 52 Inhalt A AUSGANGSLAGE: EUROPÄISCHER UND NATIONALER KONTEXT 7 B MISSION UND HANDLUNGSFELDER DER TU WIEN 8 B.1 Strategische Kooperationen und Wissenstransfer 8 B.2 Grundsätze der TU Wien 9 B.3 Handlungsfelder der TU Wien 10 C HANDLUNGSFELD „GESELLSCHAFT“ 16 C.1 Ausbau des Wissens- und Innovationstransfers 16 C.2 Unterstützung des lebensbegleitenden Wissenserwerbs 19 C.3 Heranbildung des wissenschaftlichen/künstlerischen Nachwuchses 19 C.4 Förderung von Geschlechtergerechtigkeit und Diversität 21 C.5 TU Wien – Innovativer Treiber in der digitalen Transformation 22 C.6 Erschließung von philanthropen Mitteln 23 D HANDLUNGSFELD „FORSCHUNG/ENTWICKLUNG UND ERSCHLIESSUNG DER KÜNSTE“ 25 D.1 Positionierung der TU Wien als Forschungsuniversität 25 D.2 Einrichtung von Nachwuchsgruppen 26 D.3 Kooperationen mit österreichischen Hochschul- und Forschungseinrichtungen 27 D.4 Kooperationen mit -

Energy Strategy for ETH Zurich
ESC Energy Science Center Energy Strategy for ETH Zurich ETH Zurich Energy Science Center Sonneggstrasse 3 8092 Zurich Switzerland Tel. +41 (0)44 632 83 88 www.esc.ethz.ch Imprint Scientific editors K. Boulouchos (Chair), ETH Zurich C. Casciaro, ETH Zurich K. Fröhlich, ETH Zurich S. Hellweg, ETH Zurich HJ. Leibundgut, ETH Zurich D. Spreng, ETH Zurich Layout null-oder-eins.ch Design Corporate Communications, ETH Zurich Translation and editing editranslate.com, Zurich Images Page 12, Solar Millennium AG Page 28, Axpo Available from: Energy Science Center ETH Zurich Sonneggstrasse 3 CH-8092 Zurich www.esc.ethz.ch [email protected] © Energy Science Center February 2008 Zurich Energy Strategy for ETH Zurich 1 Contents Editorial 2 Executive Summary 3 Goals of the Strategy and Working Method 8 Challenges and Boundary Conditions 9 Energy Research at ETH Zurich 13 Energy supply 14 Energy use 19 Interactions with society and the environment 24 Energy Education at ETH Zurich 29 Vision of a Transformation Path 30 Implications for ETH Zurich 35 Appendix Contributors to the Energy Strategy 39 Editorial 2 In the fall of 2006, the Energy Science Center (ESC) of The ESC members will continue to be actively involved so ETH Zurich embarked on the task of adjusting its plans that the cross-cutting strategic and operational effort for future energy-related teaching and research to match just begun here in energy research and teaching can the magnitude of the challenges in the national and glo- yield fruit. This strategy report constitutes a first impor- bal arena. At that time the executive committee of the tant step towards an intensified dialogue both within Energy Science Center instructed an internal working ETH Zurich as well as with interested partners in industry, group to begin formulating a research strategy. -
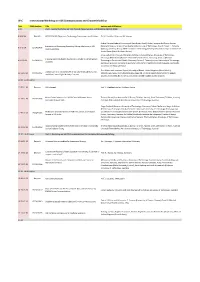
W-C International Workshop on V2X Communications and Channel Modeling
W-C International Workshop on V2X Communications and Channel Modeling Time EDAS Number Title Authors with Affiliations 9.00 Part I - Second Workshop on V2X Channel Measurements and Modeling (WVCM 2018) 9.00-9.36 Keynote H2020 5GCAR: Objectives, Technology Components and Enablers Dr. M. Condoluci, Ericsson AB, Sweden Gabriel Guieiro (Federal University of Ouro Preto, Brazil); Pedro Henrique de Oliveira Gomes Evaluation of Shadowing Caused by Mining Machinery in V2I (Federal University of Ouro Preto & Vale Institute of Technology, Brazil); Erika P. L. Almeida 9.37-9.54 1570460403 Communications (Aalborg University & INDT - Institute of Technology Development, Denmark); Luis Guilherme Uzeda Garcia (Nokia Bell Labs, France) Jonas Gedschold, Christian Schneider and Martin Käske (Ilmenau University of Technology, Germany); Mate Boban (Huawei German Research Center, Germany); Jian Luo (Huawei Tracking Based Multipath Clustering in Vehicle-to-Infrastructure 9.55-10.13 1570459752 Technologies Duesseldorf GmbH, Germany); Reiner S. Thomä (Ilmenau University of Technology, Channels Germany); Giovanni Del Galdo (Fraunhofer Institute for Integrated Circuits IIS & Technische Universität Ilmenau, Germany) Fred Wiffen and Lawrence Sayer (University of Bristol, United Kingdom (Great Britain)); Comparison of OTFS and OFDM in Ray Launched sub-6GHz and 10.13-10.30 1570463462 Mohammud Z Bocus (Toshiba Research Europe Ltd, United Kingdom (Great Britain)); Angela mmWave Line-of-Sight Mobility Channels Doufexi and Andrew Nix (University of Bristol, -

GSC-2010-Participants
REGISTRATIONS of Graduate School on Control 2010 from 01/02/2010 to 14/05/2010 Nom Prénom Service Société Ville Pays ACUNA-BRAVO Wilber Automatique informatique POLITECNICO DI TORINO TORINO ITALIA ADEGAS Fabiano D. AALBORG UUNIVERSITY Aalborg DENMARK ADEMOVIC Alma Electrical engineering University of Sarajevo Sarajevo BOSNIA ADINANDRA Sisdarmanto Eindhoven NETHERLANDS ALAWIEH Aya L2S SUPELEC Gif-sur-Yvette FRANCE ALVAREZ TORO Luz Adriana Chemical engineering USP - University of Sao Paulo Sao Paulo BRAZIL ATUONWU James Systems and control Wageningen University Wageningen NETHERLANDS BARCELLI Davide Engineering information Universita Degli Siena ITALY BAYRAK Gulden Munich GERMANY Tallinn University of BELIKOV Juri Computer control Tallinn ESTONIA Technology BENINE NETO André LIVIC Versailles FRANCE BENSMANN Boris Systemverfahrenstechnik Institut für Verfahrenstechnik Magdeburg GERMANY BEZZAOUCHA Souad L2S SUPELEC Gif sur Yvette FRANCE Eindhoven university of BIEMOND Benjamin Mechanical engineering Eindhoven NETHERLANDS technology University of California BLANDIN Sebastien Civil Systems Program BERKELEY USA Berkeley BOEGLI Max MECH-PMA Katholieke Universiteit Leuven Leuven-Heverlee BELGIUM BORRI Alessandro University of l'Aquila Monteluco di Roio ITALY BRESCH-PIETRI Delphine CAS ENSMP Neuilly/s/Marne FRANCE BREU Dominik NTNU Trondheim NORWAY BREZAS Panagiotis Cambridge UK Montbonnot Saint BRINON ARRANZ Lara Département automatique INP Grenoble FRANCE Ismier BRISTEAU Pierre-Jean CAS ENSMP Paris FRANCE Thermal processing BUCK Andreas Otto-von-Guericke -

KU Leuven KU Leuven in Short KU Leuven - History
WELCOME Prof. Paul Leroux KU Leuven KU Leuven in short KU Leuven - history . KU Leuven founded in 1425 . Oldest Catholic University in the world 2008 6 university colleges of the KU Leuven 2013 Association sign an agreement to join their KU Leuven expands to include academic educational programmes in the Associated degree programmes hosted at university Faculty of Engineering Technology and colleges within KU Leuven Association Bioscience Engineering Eminent scholars and scientists KU Leuven expands across Flanders KU Leuven in 10 locations spread over 14 campuses Leuven Sint-Lucas Campus, Ghent Group T Campus, Leuven Sint-Lucas Campus, Brussels De Nayer Campus, Sint-Katelijne Waver Brussels Campus Geel Campus Ghent, Technology Campus Carolus Campus, Antwerp Bruges Campus Sint-Andries Campus, Antwerp Kulak Campus, Kortrijk Aalst Campus Diepenbeek Campus* * The degree programme in Diepenbeek is jointly offered by Hasselt University and KU Leuven. Mission Excellence in academic education Excellence in research Distinguished service to society Programmes 78 Bachelors’s programmes 205 Master’s programmes 44 advanced Master’s programmes Characteristics Distinctive vision of education and learning Culture of quality Innovative learning environment Flexibility Internationalisation Extensive range of education facilities Figures: 2017-2018 academic year Enrolment statistics Total number of students: 56,842 Bachelor 44.5% Initial Master 31.8% Advanced Master 4.8% Doctoral Programme 10,3% Academic Teacher Training 0.6% Other 8.0% Largest student -

Chair of Controlling / Performance Management
Chair of Controlling / Performance Management Prof. Dr. Klaus Möller ANNUAL REPORT 2020 2 EDITORIAL Dear friends, supporters During the year we had quite some success in our business analytics and alumni of our chair research area: We were able to implement automated forecasts and other analytical solutions with major practice partners (like Bayer 2020 was a special year: we had a furious and Hilti) and participated at research conferences. Most notably, start followed by a lot of volatility! Most we were selected to participate in the Swiss National Research importantly we successfully managed the Program 77 about Governance of Artifical Intelligence. During the transition to intense online communica- next years, we will work intensely together with colleagues from tion due to the COVID-19 pandemic – and ETH Zurich and University of Zurich. Furthermore, we will have stayed healthy! three new PhDs from Germany, the Netherlands and the US joining us in 2021. Fortunately, we had already changed our teaching strategy and shif- ted to extensive online preparation, which paid off greatly. With the It´s a great honor and obligation that our Hilti Lab will make up help of the new team structure, integrating Assistant Prof. Dr. Maël at least 10 years of joint research, application and inspiration in Schnegg and Adjunct Prof. Dr. Matthias Mitterlechner, we reinven- the field of integrated performance management. The board of Hilti ted and redesigned all compulsory courses on bachelor and master decided to continue the sponsorship until at minimum 2023. level addressing more than 1.000 students each year. Though the publication list this year is shorter, there are two high- My dean job kept me quite busy during the whole year, not only lights on it: An A+ publication in the top journal AOS and a special with all the rearrangements in teaching due to online migration. -

Private Actors and Security Governance
Private Actors and Security Governance Alan Bryden, and Marina Caparini (editors.) © 2006, LIT & DCAF Contents List of Graphs and Tables ix Preface xi Abbreviations xiii Part I: Introduction 1 Approaching the Privatisation of Security from 3 a Security Governance Perspective Alan Bryden Part II: The International Policy Context 2 Fragile Statehood, Armed Non-State Actors 23 and Security Governance Ulrich Schneckener 3 Private Sector, Public Security 41 Alyson Bailes 4 Insurgencies, Security Governance and 65 the International Community Albrecht Schnabel. 5 Reconstructing the Public Monopoly 87 of Legitimate Force Herbert Wulf Part III: Regional and National Perspectives 6 Bulgaria's Private Security Industry 109 Philip Gounev 7 The Commercialisation of Post-Soviet Private Security 129 Duncan Hiscock 8 Challenges of Security Privatisation in Iraq 149 David Isenberg 9 Implementing South Africa’s Regulation of 167 Foreign Military Assistance Act Raenette Taljaard Part IV: Challenges of Regulation 10 Regulating Military and Security Services 189 in the European Union Elke Krahmann 11 The United Nations and the Dilemma 213 of Outsourcing Peacekeeping Operations Victor-Yves Ghebali 12 Assessing the Relationship between Humanitarian 231 Actors and Private Security Companies Christopher Spearin 13 Private Security Actors, Donors and SSR 247 Peter Wilson Part V: Conclusion 14 Applying a Security Governance Perspective to 263 the Privatisation of Security Marina Caparini Annex International Organisations and the Governance of 285 Private Security