TREATMENT B U L L E T I N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Edelgive Hurun India Philanthropy List 2020 10 November 2020
EDELGIVE HURUN INDIA PHILANTHROPY LIST 2020 10 NOVEMBER 2020 Press Release Report EdelGive Hurun India Philanthropy List 2020 Key Highlights WITH A DONATION OF INR 7,904 CRORE, AZIM PREMJI,75, WAS ‘INDIA’S MOST GENEROUS’. HE DONATED INR 22 CRORES PER DAY! HCL’S SHIV NADAR, 75, WAS SECOND WITH INR 795 CRORE DONATION WITH A DONATION OF INR 27 CRORE, AMIT CHANDRA,52, AND ARCHANA CHANDRA, 49, OF A.T.E. CHANDRA FOUNDATION ARE THE FIRST AND ONLY PROFESSIONAL MANAGERS TO EVER ENTER THE EDELGIVE HURUN INDIA PHILANTHROPY LIST. INDIAN PHILANTHROPY STATS AT A RECORD HIGH; NO. OF INDIVIDUALS WHO HAVE DONATED MORE THAN INR 10 CRORE INCREASED BY 100% OVER THE LAST 2 YEARS, FROM 37 TO 78 THIS YEAR LED BY SD SHIBULAL, 65, WHO DONATED INR 32 CRORES, 28 NEW ADDITIONS TO THE LIST; TOTAL DONATIONS BY NEW ADDITIONS AT INR 313 CRORE WITH A DONATION OF INR 458 CRORE, INDIA’S RICHEST MAN, MUKESH AMBANI,63, CAME THIRD WITH A DONATION OF INR 276 CRORE, KUMAR MANGALAM BIRLA, 53, OF ADITYA BIRLA GROUP DEBUTS THE TOP 5 AND IS THE YOUNGEST IN TOP 10 YOUNGEST: BINNY BANSAL, 37, DEBUTED THE EDELGIVE HURUN INDIA PHILANTHROPY LIST WITH A DONATION OF INR 5.3 CRORES WITH 90 PHILANTHROPISTS CUMULATIVELY DONATING INR 9,324 CRORES, EDUCATION THE MOST FAVOURED CAUSE. WITH 84 DONORS, HEALTHCARE REGISTERED A 111% INCREASE IN CUMULATIVE DONATION, FOLLOWED BY DISASTER RELIEF & MANAGEMENT, WHICH HAD 41 DONORS, REGISTERING A CUMULATIVE DONATION OF INR 354 CRORES OR AN INCREASE OF 240% 3 OF INFOSYS’S CO-FOUNDERS MADE THE LIST, WITH NANDAN NILEKANI, S GOPALAKRISHNAN AND SD SHIBULAL, -

HIV-Infected Patients
New Drugs for the Treatment-Experienced Patient Joseph Eron, md Associate Professor of Medicine and Director, Clinical Core unc Center for aids Research, University of North Carolina at Chapel Hill Summary by Tim Horn Edited by Jay Dobkin, md; Michael Saag, md reatment options for antiretro- humans by adenosine deaminase into D- Deeks and his colleagues in 1998 demon- viral-experienced patients leave a dioxolane guanine (dxg), a metabolite that strated a 1 log reduction in hiv-rna in hiv- lot to be desired. According to Dr. has potent activity against hiv and hbv. infected patients—more than 50% of whom Joe Eron, patients who have treat- According to in vitro data presented at were treatment-experienced—who received ment experience in all three classes the 3rd International Workshop on hiv tenofovir df 300 mg once daily as of currently available antiretrovi- Drug Resistance and Treatment Strategies, monotherapy for 28 days (Deeks, 1998). Trals have, at best, a 30% chance of re- held in June 1999, dapd was found to in- According to in vitro data presented by ducing their viral load to levels below 400 hibit wild-type and mutant isolates resistant Gilead’s Dr. Michael Miller at the recent copies/mL upon initiating a salvage regi- to azt (Retrovir) and 3TC (Borroto-Esoda, 4th International Resistance Workshop, the men. Cross-resistance within each class of 1999). The drug was also reported to be ac- resistance pattern for tenofovir df is simi- drugs, particularly the protease inhibitors tive against strains collected from patients lar to that of its chemical predecessor adefo- (pis) and non-nucleoside reverse tran- who have failed various nrti and nnrti vir, a compound no longer in development scriptase inhibitors (nnrtis), essentially combination therapies, including those for the treatment of hiv (Miller, 2000). -

Shifting Paradigm
SHIFTING PARADIGM How the BRICS Are Reshaping Global Health and Development ACKNOWLEDGEMENTS This report was developed by Global Health Strategies initiatives (GHSi), an international nonprofit organization advocating for improved access to health technologies and services in developing countries. Our efforts engaged the expertise of our affiliate, Global Health Strategies, an international consultancy with offices in New York, Delhi and Rio de Janeiro. This report comprises part of a larger project focused on the intersections between major growth economies and global health efforts, supported by a grant from the Bill & Melinda Gates Foundation. GHSi senior leadership, who advised the team throughout development of this report, includes David Gold and Victor Zonana (New York), Anjali Nayyar (Delhi) and Alex Menezes (Rio de Janeiro). Brad Tytel, who directs GHSi’s work on growth economies and global health, led the development of the report. Katie Callahan managed the project and led editorial efforts. Chandni Saxena supervised content development with an editorial team, including: Nidhi Dubey, Chelsea Harris, Benjamin Humphrey, Chapal Mehra, Daniel Pawson, Jennifer Payne, Brian Wahl. The research team included the following individuals, however contents do not necessarily reflect the opinions of their respective institutions: Brazil Carlos Passarelli, Senior Advisor, Treatment Advocacy, UNAIDS Cristina Pimenta, Associate Professor, School of Biological Sciences, Veiga de Almeida University Russia Kirill Danishevskiy, Assistant Professor, -

Top 200+ Current Affairs Monthly MCQ's September
Facebook Page Facebook Group Telegram Group Telegram Channel AMBITIOUSBABA.COM | ONLINE TEST SERIES: TEST.AMBITIOUSBABA.COM | MAIL 1 US AT [email protected] Facebook Page Facebook Group Telegram Group Telegram Channel Q1.India's first-ever sky cycling park to be opened in which city? (a) Manali (b) Mussoorie (c) Nainital (d) Shimla (e) None of these Ans.1.(a) Exp.To boost tourism and give and an all new experience to visitors, India's first-ever sky cycling park will soon open at Gulaba area near Manali in Himachal Pradesh. It is 350m long & is located at a height of 9000 Feet above sea level. Forest Department and Atal Bihari Vajpayee Institute of Mountaineering and Allied Sports have jointly developed an eco-friendly park. AMBITIOUSBABA.COM | ONLINE TEST SERIES: TEST.AMBITIOUSBABA.COM | MAIL 2 US AT [email protected] Facebook Page Facebook Group Telegram Group Telegram Channel Q2.Which sportsperson has won the 2019 Rashtriya Khel Protsahan Puraskar? (a) Abhinav Bindra (b) Jeev Milkha Singh (c) Mary Kom (d) Gagan Narang (e) None of these Ans.2.(d) Exp.On 2019 National Sports Day (NSD), Gagan Narang and Pawan Singh have been honoured with the Rashtriya Khel Protsahan Puraskar for their Gagan Narang Sports Promotion Foundation (GNSPF) at the Arjuna Awards ceremony in Rashtrapati Bhavan, New Delhi. The award recognizes their contribution in the growth of their favourite sport and a reward for sacrifices they have made to realize their dream. In 2011, Narang and co-founder Pawan Singh founded GNSPF to nurture budding talent -

IISER Pune Annual Report 2015-16 Chairperson Pune, India Prof
dm{f©H$ à{VdoXZ Annual Report 2015-16 ¼ããäÌãÓ¾ã ãä¶ã¹ã¥ã †Ìãâ Êãà¾ã „ÞÞã¦ã½ã ½ãÖ¦Ìã ‡ãŠñ †‡ãŠ †ñÔãñ Ìãõ—ãããä¶ã‡ãŠ ÔãâÔ©ãã¶ã ‡ãŠãè Ô©ãã¹ã¶ãã ãä•ãÔã½ãò ‚㦾ãã£ãìãä¶ã‡ãŠ ‚ã¶ãìÔãâ£ãã¶ã Ôããä֦㠂㣾ãã¹ã¶ã †Ìãâ ãäÍãàã¥ã ‡ãŠã ¹ãî¥ãùã Ôãñ †‡ãŠãè‡ãŠÀ¥ã Öãñý ãä•ã—ããÔãã ¦ã©ãã ÀÞã¶ã㦽ã‡ãŠ¦ãã Ôãñ ¾ãì§ãŠ ÔãÌããó§ã½ã Ôã½ãã‡ãŠÊã¶ã㦽ã‡ãŠ ‚㣾ãã¹ã¶ã ‡ãñŠ ½ã㣾ã½ã Ôãñ ½ããõãäÊã‡ãŠ ãäÌã—ãã¶ã ‡ãŠãñ ÀãñÞã‡ãŠ ºã¶ãã¶ããý ÊãÞããèÊãñ †Ìãâ Ôããè½ããÀãäÖ¦ã / ‚ãÔããè½ã ¹ã㟿ã‰ãŠ½ã ¦ã©ãã ‚ã¶ãìÔãâ£ãã¶ã ¹ããäÀ¾ããñ•ã¶ãã‚ããò ‡ãñŠ ½ã㣾ã½ã Ôãñ œãñ›ãè ‚ãã¾ãì ½ãò Öãè ‚ã¶ãìÔãâ£ãã¶ã àãñ¨ã ½ãò ¹ãÆÌãñÍãý Vision & Mission Establish scientific institution of the highest caliber where teaching and education are totally integrated with state-of-the- art research Make learning of basic sciences exciting through excellent integrative teaching driven by curiosity and creativity Entry into research at an early age through a flexible borderless curriculum and research projects Annual Report 2015-16 Governance Correct Citation Board of Governors IISER Pune Annual Report 2015-16 Chairperson Pune, India Prof. T.V. Ramakrishnan (till 03/12/2015) Emeritus Professor of Physics, DAE Homi Bhabha Professor, Department of Physics, Indian Institute of Science, Bengaluru Published by Dr. K. Venkataramanan (from 04/12/2015) Director and President (Engineering and Construction Projects), Dr. -
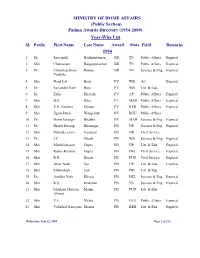
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

Khwaja Abdul Hamied
On the Margins <UN> Muslim Minorities Editorial Board Jørgen S. Nielsen (University of Copenhagen) Aminah McCloud (DePaul University, Chicago) Jörn Thielmann (Erlangen University) volume 34 The titles published in this series are listed at brill.com/mumi <UN> On the Margins Jews and Muslims in Interwar Berlin By Gerdien Jonker leiden | boston <UN> This is an open access title distributed under the terms of the CC BY-NC-ND 4.0 license, which permits any non-commercial use, distribution, and reproduction in any medium, provided no alterations are made and the original author(s) and source are credited. Further information and the complete license text can be found at https://creativecommons.org/licenses/by-nc-nd/4.0/ The terms of the CC license apply only to the original material. The use of material from other sources (indicated by a reference) such as diagrams, illustrations, photos and text samples may require further permission from the respective copyright holder. Cover illustration: The hiking club in Grunewald, 1934. PA Oettinger, courtesy Suhail Ahmad. Library of Congress Cataloging-in-Publication Data Names: Jonker, Gerdien, author. Title: On the margins : Jews and Muslims in interwar Berlin / by Gerdien Jonker. Description: Leiden ; Boston : Brill, [2020] | Series: Muslim minorities, 1570–7571 ; volume 34 | Includes bibliographical references and index. Identifiers: LCCN 2019051623 (print) | LCCN 2019051624 (ebook) | ISBN 9789004418738 (hardback) | ISBN 9789004421813 (ebook) Subjects: LCSH: Jews--Germany--Berlin--Social conditions--20th century. | Muslims--Germany--Berlin--Social conditions--20th century. | Muslims --Cultural assimilation--Germany--Berlin. | Jews --Cultural assimilation --Germany--Berlin. | Judaism--Relations--Islam. | Islam --Relations--Judaism. | Social integration--Germany--Berlin. -

(12) Patent Application Publication (10) Pub. No.: US 2016/0058872 A1 Crew Et Al
US 2016.0058872A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0058872 A1 Crew et al. (43) Pub. Date: Mar. 3, 2016 (54) IMIDE-BASED MODULATORS OF A613 L/426 (2006.01) PROTEOLYSIS AND ASSOCATED METHODS A 6LX3 L/505 (2006.01) OF USE A613 L/454 (2006.01) A613 L/55 (2006.01) (71) Applicant: Arvinas, Inc., New Haven, CT (US) C07D40 L/4 (2006.01) A6II 45/06 (2006.01) (72) Inventors: Andrew P. Crew, Guilford, CT (US); (52) U.S. Cl. Craig Crews, New Haven, CT (US), CPC ............ A61K 47/481 (2013.01); C07D401/14 Hanging Dong, Madison, CT (US); Jing (2013.01); C07D 495/14 (2013.01); C07D Wang, Milford, CT (US); Yimin Qian, 417/14 (2013.01); A61K 45/06 (2013.01); Plainsboro, NJ (US); Kam Siu Milford, A6 IK3I/505 (2013.01); A61 K3I/454 CT (US); Meizhong Jin, East Northport, (2013.01); A61 K3I/551 (2013.01); A61 K NY (US) 3 1/426 (2013.01) (21) Appl. No.: 14/792,414 (57) ABSTRACT (22) Filed: Jul. 6, 2015 The description relates to imide-based compounds, including Related U.S. Application Data bifunctional compounds comprising the same, which find utility as modulators of targeted ubiquitination, especially (63) Continuation-in-part of application No. 14/686.640, inhibitors of a variety of polypeptides and other proteins filed on Apr. 14, 2015. which are degraded and/or otherwise inhibited by bifunc (60) Provisional application No. 61/979,351, filed on Apr. tional compounds according to the present invention. In par 14, 2014, provisional application No. -

India Emerging As an Economic Superpower
IOSR Journal Of Humanities And Social Science (IOSR-JHSS) Volume 20, Issue 5, Ver. IV (May. 2015), PP 45-50 e-ISSN: 2279-0837, p-ISSN: 2279-0845. www.iosrjournals.org India Emerging as an Economic Superpower Himani Assistant Professor In Economics D.A.V. College For Girls, Yamuna Nagar Abstract: With nearly 1.1 billion inhabitants, India is the second largest country on earth in population, and seventh largest in geographical area, over 1.1 million square miles. This is almost 1,000 people for every square mile of area nationwide—much denser than even China. Since achieving independence from British rule in 1947, it has seen its share of conflict, struggle and setbacks. Although India still faces many challenges, it is now poised to reach a higher position on the world scene than at any previous time. The Indian economy has grown an average of around 6% annually over the past decade and 8% per year over the past three years— among the fastest rates in the world. It boasts an emerging middle class and increasing gross domestic product, exports, employment and foreign investment. This is complemented by a roaring stock market (index value up by a third in 2005 and by 200% since 2001), low external debt and large foreign exchange reserves. Recent visits from leaders and officials from the United States, France, Germany and Russia have spotlighted India‟s rise. These wealthier nations see India as a trading partner with enormous potential. Now the question is „Will India Become a Superpower?‟ This paper is an attempt to show that “Whether India is really becoming an economic super power or is it a myth?” I. -

Modifications to the Harmonized Tariff Schedule of the United States To
U.S. International Trade Commission COMMISSIONERS Shara L. Aranoff, Chairman Daniel R. Pearson, Vice Chairman Deanna Tanner Okun Charlotte R. Lane Irving A. Williamson Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement the Dominican Republic- Central America-United States Free Trade Agreement With Respect to Costa Rica Publication 4038 December 2008 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 18, 2008, set forth in the Appendix hereto, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement the Dominican Republic- Central America-United States Free Trade Agreement, as approved in the Dominican Republic-Central America- United States Free Trade Agreement Implementation Act, with respect to Costa Rica. (This page is intentionally blank) Annex I Effective with respect to goods that are entered, or withdrawn from warehouse for consumption, on or after January 1, 2009, the Harmonized Tariff Schedule of the United States (HTS) is modified as provided herein, with bracketed matter included to assist in the understanding of proclaimed modifications. The following supersedes matter now in the HTS. (1). General note 4 is modified as follows: (a). by deleting from subdivision (a) the following country from the enumeration of independent beneficiary developing countries: Costa Rica (b). -

CGC | Mumbai Annual Report 2018-19
ANNUAL REPORT 2018 - 2019 ANNUAL REPORT | SEPTEMBER 2018 - AUGUST 2019 CONTENTS 05 MESSAGE FROM THE DIRECTOR 06 ADVISORY BOARD 07 FACULTY ADVISORY COMMITTEE 08 WHAT WE DO 09-11 PUBLIC PROGRAMS “The most pressing issues we are grappling with today – increasing 12-13 STUDENT PROGRAMS political polarization, accelerating climate change, deepening 14 - 15 RESEARCH PROJECTS inequality – are inherently global in nature. Understanding their 16 KEY STATISTICS impact, and formulating intelligent responses, is impossible without sustained engagement in and with 17-30 PROGRAM HIGHLIGHTS the world. This is precisely why the Columbia Global Centers were 18 -21 Innovation and Entrepreneurship created ten years ago – to be MENTORING SOCIAL IMPACT STARTUPS deeply responsive to and integrated with issues of local, 22 - 24 Health and Medicine regional, and global significance. RESEARCHING CRITICAL HEALTH ISSUES By allowing us to learn from and with the world, the Centers advance knowledge and its 25 - 27 Environmental Sustainability exchange, helping us to study CONSERVING URBAN BIODIVERSITY significant questions and address the most urgent global challenges.” 28-30 Education and Culture STRENGTHENING HIGHER EDUCATION SAFWAN M. MASRI Executive Vice President for Global 31 COLUMBIA GLOBAL CENTERS | MUMBAI TEAM Centers and Global Development at Columbia University MESSAGE FROM THE DIRECTOR As India and the world grapple with the consequences of environmental degradation and climate change, as well as major shifts in pluralism and democracy, questions around what research and education mean, and what shape next generation knowledge must take, become even more critical. At the Columbia Global Center | Mumbai, we remain committed to the vision of building academic bridges for a better future. -

(12) Patent Application Publication (10) Pub. No.: US 2015/0250896 A1 Zhao (43) Pub
US 20150250896A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0250896 A1 Zhao (43) Pub. Date: Sep. 10, 2015 (54) HYDROPHILIC LINKERS AND THEIR USES Publication Classification FOR CONUGATION OF DRUGS TO A CELL (51) Int. Cl BNDING MOLECULES A647/48 (2006.01) (71) Applicant: Yongxin R. ZHAO, Henan (CN) Ek E. 30.8 C07D 207/216 (2006.01) (72) Inventor: R. Yongxin Zhao, Lexington, MA (US) C07D 40/12 (2006.01) C07F 9/30 (2006.01) C07F 9/572 (2006.01) (73) Assignee: Hangzhou DAC Biotech Co., Ltd., (52) U.S. Cl. Hangzhou City, ZJ (CN) CPC ........... A61K47/48715 (2013.01); C07F 9/301 (2013.01); C07F 9/65583 (2013.01); C07F (21) Appl. No.: 14/432,073 9/5721 (2013.01); C07D 207/46 (2013.01); C07D 401/12 (2013.01); A61 K3I/454 (22) PCT Filed: Nov. 24, 2012 (2013.01) (86). PCT No.: PCT/B2O12/0567OO Cell(57) binding- agent-drugABSTRACT conjugates comprising hydrophilic- S371 (c)(1), linkers, and methods of using Such linkers and conjugates are (2) Date: Mar. 27, 2015 provided. Patent Application Publication Sep. 10, 2015 Sheet 1 of 23 US 2015/0250896 A1 O HMDS OSiMe 2n O Br H-B-H HPC 3 2 COOEt essiop-\5. E B to NH 120 °C, 2h OsiMe3 J 50 °C, 2h eSiO OEt 120 oC, sh 1 2 3. 42% from 1 Bra-11a1'oet - Brn 11-1 or a 1-1 or ÓH 140 °C ÓEt ÓEt 4 5 6 - --Messio. 8 B1a-Br aus 20 cc, hP-1}^-'ot Br1-Y.