02 Geometry, Reclattice.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Reciprocal Lattice
Appendix A THE RECIPROCAL LATTICE When the translations of a primitive space lattice are denoted by a, b and c, the vector p to any lattice point is given p = ua + vb + we. The definition of the reciprocal lattice is that the translations a*, b* and c*, which define the reciprocal lattice fulfil the following relationships: (A. I) a*.b = b*.c = c*.a = a.b* = b.c* =c. a*= 0 (A.2) It can then be easily shown that: (1) Ic* I = 1/c-spacing of primitive lattice and similarly forb* and a*. (2) a*= (b 1\ c)ja.(b 1\ c) b* = (c 1\ a)jb.(c 1\ a) c* =(a 1\ b)/c-(a 1\ b) These last three relations are often used as a definition of the reciprocal lattice. Two properties of the reciprocal lattice are particularly important: (a) The vector g* defined by g* = ha* + kb* + lc* (where h, k and I are integers to the point hkl in the reciprocal lattice) is normal to the plane of Miller indices (hkl) in the primary lattice. (b) The magnitude Ig* I of this vector is the reciprocal of the spacing of (hkl) in the primary lattice. AI ALLOWED REFLECTIONS The kinematical structure factor for reflections is given by Fhkl = I;J;exp[- 2ni(hu; + kv; + lw)] (A.3) where ui' v; and W; are the coordinates of the atoms and hkl the Miller indices of the reflection g. If there is only one atom at 0, 0, 0 in the unit cell then the structure factor will be independent of hkl since for all values of h, k and I we have Fhkl =f. -

The 4 Reciprocal Lattice
4 The Reciprocal Lattice by Andr~ Authier This electronic edition may be freely copied and redistributed for educational or research purposes only. It may not be sold for profit nor incorporated in any product sold for profit without the express pernfission of The Executive Secretary, International Union of Crystallography, 2 Abbey Square, Chester CIII 211[;, [;K Copyright in this electronic ectition (<.)2001 International l.Jnion of Crystallography Published for the International Union of Crystallography by University College Cardiff Press Cardiff, Wales © 1981 by the International Union of Crystallography. All rights reserved. Published by the University College Cardiff Press for the International Union of Crystallography with the financial assistance of Unesco Contract No. SC/RP 250.271 This pamphlet is one of a series prepared by the Commission on Crystallographic Teaching of the International Union of Crystallography, under the General Editorship of Professor C. A. Taylor. Copies of this pamphlet and other pamphlets in the series may be ordered direct from the University College Cardiff Press, P.O. Box 78, Cardiff CF1 1XL, U.K. ISBN 0 906449 08 I Printed in Wales by University College, Cardiff. Series Preface The long term aim of the Commission on Crystallographic Teaching in establishing this pamphlet programme is to produce a large collection of short statements each dealing with a specific topic at a specific level. The emphasis is on a particular teaching approach and there may well, in time, be pamphlets giving alternative teaching approaches to the same topic. It is not the function of the Commission to decide on the 'best' approach but to make all available so that teachers can make their own selection. -

Crystal Structure of a Material Is Way in Which Atoms, Ions, Molecules Are Spatially Arranged in 3-D Space
Crystalline Structures – The Basics •Crystal structure of a material is way in which atoms, ions, molecules are spatially arranged in 3-D space. •Crystal structure = lattice (unit cell geometry) + basis (atom, ion, or molecule positions placed on lattice points within the unit cell). •A lattice is used in context when describing crystalline structures, means a 3-D array of points in space. Every lattice point must have identical surroundings. •Unit cell: smallest repetitive volume •Each crystal structure is built by stacking which contains the complete lattice unit cells and placing objects (motifs, pattern of a crystal. A unit cell is chosen basis) on the lattice points: to represent the highest level of geometric symmetry of the crystal structure. It’s the basic structural unit or building block of crystal structure. 7 crystal systems in 3-D 14 crystal lattices in 3-D a, b, and c are the lattice constants 1 a, b, g are the interaxial angles Metallic Crystal Structures (the simplest) •Recall, that a) coulombic attraction between delocalized valence electrons and positively charged cores is isotropic (non-directional), b) typically, only one element is present, so all atomic radii are the same, c) nearest neighbor distances tend to be small, and d) electron cloud shields cores from each other. •For these reasons, metallic bonding leads to close packed, dense crystal structures that maximize space filling and coordination number (number of nearest neighbors). •Most elemental metals crystallize in the FCC (face-centered cubic), BCC (body-centered cubic, or HCP (hexagonal close packed) structures: Room temperature crystal structure Crystal structure just before it melts 2 Recall: Simple Cubic (SC) Structure • Rare due to low packing density (only a-Po has this structure) • Close-packed directions are cube edges. -

Types of Lattices
Types of Lattices Types of Lattices Lattices are either: 1. Primitive (or Simple): one lattice point per unit cell. 2. Non-primitive, (or Multiple) e.g. double, triple, etc.: more than one lattice point per unit cell. Double r2 cell r1 r2 Triple r1 cell r2 r1 Primitive cell N + e 4 Ne = number of lattice points on cell edges (shared by 4 cells) •When repeated by successive translations e =edge reproduce periodic pattern. •Multiple cells are usually selected to make obvious the higher symmetry (usually rotational symmetry) that is possessed by the 1 lattice, which may not be immediately evident from primitive cell. Lattice Points- Review 2 Arrangement of Lattice Points 3 Arrangement of Lattice Points (continued) •These are known as the basis vectors, which we will come back to. •These are not translation vectors (R) since they have non- integer values. The complexity of the system depends upon the symmetry requirements (is it lost or maintained?) by applying the symmetry operations (rotation, reflection, inversion and translation). 4 The Five 2-D Bravais Lattices •From the previous definitions of the four 2-D and seven 3-D crystal systems, we know that there are four and seven primitive unit cells (with 1 lattice point/unit cell), respectively. •We can then ask: can we add additional lattice points to the primitive lattices (or nets), in such a way that we still have a lattice (net) belonging to the same crystal system (with symmetry requirements)? •First illustrate this for 2-D nets, where we know that the surroundings of each lattice point must be identical. -

The Metrical Matrix in Teaching Mineralogy G. V
THE METRICAL MATRIX IN TEACHING MINERALOGY G. V. GIBBS Department of Geological Sciences & Department of Materials Science and Engineering Virginia Polytechnic Institute and State University Blacksburg, VA 24061 [email protected] INTRODUCTION The calculation of the d-spacings, the angles between planes and zones, the bond lengths and angles and other important geometric relationships for a mineral can be a tedious task both for the student and the instructor, particularly when completed with the large as- sortment of trigonometric identities and algebraic formulae that are available (d. Crystal Geometry (1959), Donnay and Donnay, International Tables for Crystallography, Vol. II, Section 3, The Kynoch Press, 101-158). However, such calculations are straightforward and relatively easy to do when completed with the metrical matrix and the interactive software MATOP. Several applications of the matrix are presented below, each of which is worked out in detail and which is designed to teach you its use in the study of crystal geometry. SOME PRELIMINARY COMMENTS We begin our discussion of the matrix with a brief examination of the properties of the geometric three dimensional space, S, in which we live and in which minerals and rocks occur. For our purposes, it will be convenient to view S as the set of all vectors that radiate from a common origin to each point in space. In constructing a model for S, we chose three noncoplanar, coordinate axes denoted X, Y and Z, each radiating from the origin, O. Next, we place three nonzero vectors denoted a, band c along X, Y and Z, respectively, likewise radiating from O. -
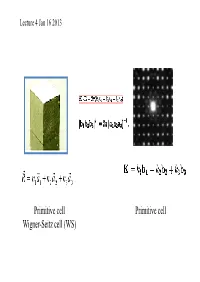
Primitive Cell Wigner-Seitz Cell (WS) Primitive Cell
Lecture 4 Jan 16 2013 Primitive cell Primitive cell Wigner-Seitz cell (WS) First Brillouin zone The Wigner-Seitz primitive cell of the reciprocal lattice is known as the first Brillouin zone. (Wigner-Seitz is real space concept while Brillouin zone is a reciprocal space idea). Powder cell Polymorphic Forms of Carbon Graphite – a soft, black, flaky solid, with a layered structure – parallel hexagonal arrays of carbon atoms – weak van der Waal’s forces between layers – planes slide easily over one another Miller indices Simple cubic Miller Indices Rules for determining Miller Indices: 1. Determine the intercepts of the face along the crystallographic axes, in terms of un it ce ll dimens ions. 2. Take the reciprocals 3. Clear fractions 4. Reduce to lowest terms Simple cubic d100=? Where does a protein crystallographer see the Miller indices? CtlCommon crystal faces are parallel to lattice planes • Eac h diffrac tion spo t can be regarded as a X-ray beam reflected from a lattice plane , and therefore has a unique Miller index. Miller indices A Miller index is a series of coprime integers that are inversely ppproportional to the intercepts of the cry stal face or crystallographic planes with the edges of the unit cell. It describes the orientation of a plane in the 3-D lattice with respect to the axes. The general form of the Miller index is (h, k, l) where h, k, and l are integers related to the unit cell along the a, b, c crystal axes. Irreducible brillouin zone II II II II Reciprocal lattice ghakblc The Bravais lattice after Fourier transform real space reciprocal lattice normaltthlls to the planes (vect ors ) poitints spacing between planes 1/distance between points ((y,p)actually, 2p/distance) l (distance, wavelength) 2p/l=k (momentum, wave number) BillBravais cell Wigner-SiSeitz ce ll Brillouin zone . -

Chapter 2 X-Ray Diffraction and Reciprocal Lattice
Chapter 2 X-ray diffraction and reciprocal lattice I. Waves 1. A plane wave is described as Ψ(x,t) = A ei(k⋅x-ωt) A is the amplitude, k is the wave vector, and ω=2πf is the angular frequency. 2. The wave is traveling along the k direction with a velocity c given by ω=c|k|. Wavelength along the traveling direction is given by |k|=2π/λ. 3. When a wave interacts with the crystal, the plane wave will be scattered by the atoms in a crystal and each atom will act like a point source (Huygens’ principle). 4. This formulation can be applied to any waves, like electromagnetic waves and crystal vibration waves; this also includes particles like electrons, photons, and neutrons. A particular case is X-ray. For this reason, what we learn in X-ray diffraction can be applied in a similar manner to other cases. II. X-ray diffraction in real space – Bragg’s Law 1. A crystal structure has lattice and a basis. X-ray diffraction is a convolution of two: diffraction by the lattice points and diffraction by the basis. We will consider diffraction by the lattice points first. The basis serves as a modification to the fact that the lattice point is not a perfect point source (because of the basis). 2. If each lattice point acts like a coherent point source, each lattice plane will act like a mirror. θ θ θ d d sin θ (hkl) -1- 2. The diffraction is elastic. In other words, the X-rays have the same frequency (hence wavelength and |k|) before and after the reflection. -

Chapter 4, Bravais Lattice Primitive Vectors
Chapter 4, Bravais Lattice A Bravais lattice is the collection of all (and only those) points in space reachable from the origin with position vectors: n , n , n integer (+, -, or 0) r r r r 1 2 3 R = n1a1 + n2 a2 + n3a3 a1, a2, and a3 not all in same plane The three primitive vectors, a1, a2, and a3, uniquely define a Bravais lattice. However, for one Bravais lattice, there are many choices for the primitive vectors. A Bravais lattice is infinite. It is identical (in every aspect) when viewed from any of its lattice points. This is not a Bravais lattice. Honeycomb: P and Q are equivalent. R is not. A Bravais lattice can be defined as either the collection of lattice points, or the primitive translation vectors which construct the lattice. POINT Q OBJECT: Remember that a Bravais lattice has only points. Points, being dimensionless and isotropic, have full spatial symmetry (invariant under any point symmetry operation). Primitive Vectors There are many choices for the primitive vectors of a Bravais lattice. One sure way to find a set of primitive vectors (as described in Problem 4 .8) is the following: (1) a1 is the vector to a nearest neighbor lattice point. (2) a2 is the vector to a lattice points closest to, but not on, the a1 axis. (3) a3 is the vector to a lattice point nearest, but not on, the a18a2 plane. How does one prove that this is a set of primitive vectors? Hint: there should be no lattice points inside, or on the faces (lll)fhlhd(lllid)fd(parallolegrams) of, the polyhedron (parallelepiped) formed by these three vectors. -

Lecture Notes
Solid State Physics PHYS 40352 by Mike Godfrey Spring 2012 Last changed on May 22, 2017 ii Contents Preface v 1 Crystal structure 1 1.1 Lattice and basis . .1 1.1.1 Unit cells . .2 1.1.2 Crystal symmetry . .3 1.1.3 Two-dimensional lattices . .4 1.1.4 Three-dimensional lattices . .7 1.1.5 Some cubic crystal structures ................................ 10 1.2 X-ray crystallography . 11 1.2.1 Diffraction by a crystal . 11 1.2.2 The reciprocal lattice . 12 1.2.3 Reciprocal lattice vectors and lattice planes . 13 1.2.4 The Bragg construction . 14 1.2.5 Structure factor . 15 1.2.6 Further geometry of diffraction . 17 2 Electrons in crystals 19 2.1 Summary of free-electron theory, etc. 19 2.2 Electrons in a periodic potential . 19 2.2.1 Bloch’s theorem . 19 2.2.2 Brillouin zones . 21 2.2.3 Schrodinger’s¨ equation in k-space . 22 2.2.4 Weak periodic potential: Nearly-free electrons . 23 2.2.5 Metals and insulators . 25 2.2.6 Band overlap in a nearly-free-electron divalent metal . 26 2.2.7 Tight-binding method . 29 2.3 Semiclassical dynamics of Bloch electrons . 32 2.3.1 Electron velocities . 33 2.3.2 Motion in an applied field . 33 2.3.3 Effective mass of an electron . 34 2.4 Free-electron bands and crystal structure . 35 2.4.1 Construction of the reciprocal lattice for FCC . 35 2.4.2 Group IV elements: Jones theory . 36 2.4.3 Binding energy of metals . -

Introduction to Higher Dimensional Description of Quasicrystal Structures
ISQCS, June 23-27, 2019, Sendai, Tohoku University Introduction to higher dimensional description of quasicrystal structures Hiroyuki Takakura Division of Applied Physics, Faculty of Engineering, Hokkaido University ISQCS, June 23-27, 2019, Sendai, Tohoku University Outline • Diffraction symmetries & Space groups of iQCs • Section method • Fibonacci structure • Icosahedral lattices • Simple models of iQCs • Real iQC structures • Cluster based model of iQCs • Summary ISQCS, June 23-27, 2019, Sendai, Tohoku University Crystal Amorphous Their diffraction patterns ISQCS, June 23-27, 2019, Sendai, Tohoku University Diffraction symmetries and space groups of iQCs ISQCS, June 23-27, 2019, Sendai, Tohoku University X-ray transmission Laue patterns of iQC 2-fold 3-fold 5-fold i-Zn-Mg-Ho F-type ISQCS, June 23-27, 2019, Sendai, Tohoku University Electron diffraction pattern of iQC i-AlMn 1 The arrangement of the diffraction spots is not periodic but quasi-periodic. D.Shechtman et al., Phys.Rev.Lett., 53,1951(1984). ISQCS, June 23-27, 2019, Sendai, Tohoku University Symmetry of iQC 2 Point group 31.72º 5 Order : 120 37.38º 2 5 3 20.90º 3 2 2 Asymmetric region: 6 +10 +15 + m + center ISQCS, June 23-27, 2019, Sendai, Tohoku University X-ray diffraction patterns of iQCs P-type i-Zn-Mg-Ho F-type i-Zn-Mg-Ho 2fy 2fy 5f 5f 3f 3f 2fx 2fx Liner plots ISQCS, June 23-27, 2019, Sendai, Tohoku University X-ray diffraction patterns of iQCs P-type i-Zn-Mg-Ho F-type i-Zn-Mg-Ho 2fy 2fy 5f 5f 3f 3f 2fx All even or all odd for 2fx No reflection condition Log plots ISQCS, June 23-27, 2019, Sendai, Tohoku University Vectors used for indexing 6 Any vectors can be used if all the reflections can be indexed correctly. -

Symmetry and Groups, and Crystal Structures
CHAPTER 3: SYMMETRY AND GROUPS, AND CRYSTAL STRUCTURES Sarah Lambart RECAP CHAP. 2 2 different types of close packing: hcp: tetrahedral interstice (ABABA) ccp: octahedral interstice (ABCABC) Definitions: The coordination number or CN is the number of closest neighbors of opposite charge around an ion. It can range from 2 to 12 in ionic structures. These structures are called coordination polyhedron. RECAP CHAP. 2 Rx/Rz C.N. Type Hexagonal or An ideal close-packing of sphere 1.0 12 Cubic for a given CN, can only be Closest Packing achieved for a specific ratio of 1.0 - 0.732 8 Cubic ionic radii between the anions and 0.732 - 0.414 6 Octahedral Tetrahedral (ex.: the cations. 0.414 - 0.225 4 4- SiO4 ) 0.225 - 0.155 3 Triangular <0.155 2 Linear RECAP CHAP. 2 Pauling’s rule: #1: the coodination polyhedron is defined by the ratio Rcation/Ranion #2: The Electrostatic Valency (e.v.) Principle: ev = Z/CN #3: Shared edges and faces of coordination polyhedra decreases the stability of the crystal. #4: In crystal with different cations, those of high valency and small CN tend not to share polyhedral elements #5: The principle of parsimony: The number of different sites in a crystal tends to be small. CONTENT CHAP. 3 (2-3 LECTURES) Definitions: unit cell and lattice 7 Crystal systems 14 Bravais lattices Element of symmetry CRYSTAL LATTICE IN TWO DIMENSIONS A crystal consists of atoms, molecules, or ions in a pattern that repeats in three dimensions. The geometry of the repeating pattern of a crystal can be described in terms of a crystal lattice, constructed by connecting equivalent points throughout the crystal. -

Quasicrystals
Volume 106, Number 6, November–December 2001 Journal of Research of the National Institute of Standards and Technology [J. Res. Natl. Inst. Stand. Technol. 106, 975–982 (2001)] Quasicrystals Volume 106 Number 6 November–December 2001 John W. Cahn The discretely diffracting aperiodic crystals Key words: aperiodic crystals; new termed quasicrystals, discovered at NBS branch of crystallography; quasicrystals. National Institute of Standards and in the early 1980s, have led to much inter- Technology, disciplinary activity involving mainly Gaithersburg, MD 20899-8555 materials science, physics, mathematics, and crystallography. It led to a new un- Accepted: August 22, 2001 derstanding of how atoms can arrange [email protected] themselves, the role of periodicity in na- ture, and has created a new branch of crys- tallography. Available online: http://www.nist.gov/jres 1. Introduction The discovery of quasicrystals at NBS in the early Crystal periodicity has been an enormously important 1980s was a surprise [1]. By rapid solidification we had concept in the development of crystallography. Hau¨y’s made a solid that was discretely diffracting like a peri- hypothesis that crystals were periodic structures led to odic crystal, but with icosahedral symmetry. It had long great advances in mathematical and experimental crys- been known that icosahedral symmetry is not allowed tallography in the 19th century. The foundation of crys- for a periodic object [2]. tallography in the early nineteenth century was based on Periodic solids give discrete diffraction, but we did the restrictions that periodicity imposes. Periodic struc- not know then that certain kinds of aperiodic objects can tures in two or three dimensions can only have 1,2,3,4, also give discrete diffraction; these objects conform to a and 6 fold symmetry axes.