IDENTIFICATION of HPHT-TREATED YELLOW to GREEN DIAMONDS by Ilene M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
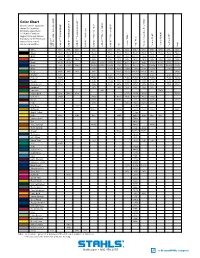
Color Chart ® ® ® ® Closest Pantone® Equivalent Shown
™ ™ II ® Color Chart ® ® ® ® Closest Pantone® equivalent shown. Due to printing limitations, colors shown 5807 Reflective ® ® ™ ® ® and Pantone numbers ® ™ suggested may vary from ac- ECONOPRINT GORILLA GRIP Fashion-REFLECT Reflective Thermo-FILM Thermo-FLOCK Thermo-GRIP ® ® ® ® ® ® ® tual colors. For the truest color ® representation, request Scotchlite our material swatches. ™ CAD-CUT 3M CAD-CUT CAD-CUT CAD-CUT CAD-CUT CAD-CUT CAD-CUT Felt Perma-TWILL Poly-TWILL Thermo-FILM Thermo-FLOCK Thermo-GRIP Vinyl Pressure Sensitive Poly-TWILL Sensitive Pressure CAD-CUT White White White White White White White White White* White White White White White Black Black Black Black Black Black Black Black Black* Black Black Black Black Black Gold 1235C 136C 137C 137C 123U 715C 1375C* 715C 137C 137C 116U Red 200C 200C 703C 186C 186C 201C 201C 201C* 201C 186C 186C 186C 200C Royal 295M 294M 7686C 2747C 7686C 280C 294C 294C* 294C 7686C 2758C 7686C 654C Navy 296C 2965C 7546C 5395M 5255C 5395M 276C 532C 532C* 532C 5395M 5255C 5395M 5395C Cool Gray Warm Gray Gray 7U 7539C 7539C 415U 7538C 7538C* 7538C 7539C 7539C 2C Kelly 3415C 341C 340C 349C 7733C 7733C 7733C* 7733C 349C 3415C Orange 179C 1595U 172C 172C 7597C 7597C 7597C* 7597C 172C 172C 173C Maroon 7645C 7645C 7645C Black 5C 7645C 7645C* 7645C 7645C 7645C 7449C Purple 2766C 7671C 7671C 669C 7680C 7680C* 7680C 7671C 7671C 2758U Dark Green 553C 553C 553C 447C 567C 567C* 567C 553C 553C 553C Cardinal 201C 188C 195C 195C* 195C 201C Emerald 348 7727C Vegas Gold 616C 7502U 872C 4515C 4515C 4515C 7553U Columbia 7682C 7682C 7459U 7462U 7462U* 7462U 7682C Brown Black 4C 4675C 412C 412C Black 4C 412U Pink 203C 5025C 5025C 5025C 203C Mid Blue 2747U 2945U Old Gold 1395C 7511C 7557C 7557C 1395C 126C Bright Yellow P 4-8C Maize 109C 130C 115U 7408C 7406C* 7406C 115U 137C Canyon Gold 7569C Tan 465U Texas Orange 7586C 7586C 7586C Tenn. -

Color Chart Colorchart
Color Chart AMERICANA ACRYLICS Snow (Titanium) White White Wash Cool White Warm White Light Buttermilk Buttermilk Oyster Beige Antique White Desert Sand Bleached Sand Eggshell Pink Chiffon Baby Blush Cotton Candy Electric Pink Poodleskirt Pink Baby Pink Petal Pink Bubblegum Pink Carousel Pink Royal Fuchsia Wild Berry Peony Pink Boysenberry Pink Dragon Fruit Joyful Pink Razzle Berry Berry Cobbler French Mauve Vintage Pink Terra Coral Blush Pink Coral Scarlet Watermelon Slice Cadmium Red Red Alert Cinnamon Drop True Red Calico Red Cherry Red Tuscan Red Berry Red Santa Red Brilliant Red Primary Red Country Red Tomato Red Naphthol Red Oxblood Burgundy Wine Heritage Brick Alizarin Crimson Deep Burgundy Napa Red Rookwood Red Antique Maroon Mulberry Cranberry Wine Natural Buff Sugared Peach White Peach Warm Beige Coral Cloud Cactus Flower Melon Coral Blush Bright Salmon Peaches 'n Cream Coral Shell Tangerine Bright Orange Jack-O'-Lantern Orange Spiced Pumpkin Tangelo Orange Orange Flame Canyon Orange Warm Sunset Cadmium Orange Dried Clay Persimmon Burnt Orange Georgia Clay Banana Cream Sand Pineapple Sunny Day Lemon Yellow Summer Squash Bright Yellow Cadmium Yellow Yellow Light Golden Yellow Primary Yellow Saffron Yellow Moon Yellow Marigold Golden Straw Yellow Ochre Camel True Ochre Antique Gold Antique Gold Deep Citron Green Margarita Chartreuse Yellow Olive Green Yellow Green Matcha Green Wasabi Green Celery Shoot Antique Green Light Sage Light Lime Pistachio Mint Irish Moss Sweet Mint Sage Mint Mint Julep Green Jadeite Glass Green Tree Jade -

BWSR Featured Plant: Downy Yellow Violet
2019 June Plant of the Month BWSR Featured Plant Name: Downy yellow violet (Viola pubescens) Plant family: Violet (Violaceae) Downy yellow Downy yellow violet, AKA hairy yellow violets are an important early violet or smooth yellow violet, is food source for a short (4- to 12-inch-tall), native, pollinators. Fine hairs along the herbaceous perennial that blooms in rounded teeth woodlands, gardens edge of the leaf are a distinguishing and shady areas Plant Stats feature. Brown lines starting in April. It STATEWIDE on the flower petals provides an early lead pollinators to WETLAND nectar and pollen. splash of color and INDICATOR Photo Credits: important early STATUS: FACU Heather Holm season nectar and PRIMARY USES: pollen. Like other Ground cover, shade/pollinator Viola species, this gardens, edibles, plant produces both woodland showy, open cross- restorations pollinating flowers at the top of the plant, and fully closed, self-pollinating flowers that may be found aboveground or underground. The showy flowers bloom before trees leaf out. The closed flowers bloom once the tree canopy leafs out. Planting Recommendations Range Downy yellow violets and can be used as Downy yellow violet is found may not be as an alternative to turf throughout Minnesota. Records aggressive as some grass, along paths and exist in all but a handful of other violets in a woodland borders, counties. It is mostly found east garden, but will spread and can be mixed with of the Missouri River, with a few over time in ideal other short woodland records west of the Missouri. conditions — part plants such as sedges, Its range stretches into New shade and medium to anemones and wild England and north into central dry soils. -

Red Yellow Blue Worksheets (Pdf)
Red Yellow Blue By Lysa Mullady Follow Up Activity and Worksheets for Elementary Students Red Yellow Blue By Lysa Mullady Red Yellow Blue is a book that supports social and emotional learning. It explores a wide range of feelings and the importance of cooperation. When discussing feelings in the classroom, it is important to: § Validate that all feelings are ok. § Teach the importance of talking out your feelings with a trusted person as a means of coping with difficult emotions. § Make the connection between the emotion and the event that caused it. Cooperation is the action of working together towards a common goal. Working together makes a job easier and gives everyone involved positive feelings of accomplishment and belonging. Kids show cooperation by: § Listening not only to instructions, but to other members of the group. § Being wiling to take turns and share when necessary. § Being able to compromise, to change their point of view based on the ideas of the group. § Using encouraging words. § Appreciating what others do. Red Yellow Blue Paper Chain Cooperation Class Activity Objectives: § The students will be able to define cooperation. § The students will be able to list actions that show cooperation. § The students will be able to work demonstrate cooperation by working together to create a paper chain. Materials: Copy and cut our the strips of paper. Each student should receive three strips, one with each character. If possible, make copies on red, yellow and blue paper or have the students color the strips red, yellow and blue. You will also need chart paper or a white board to list student responses. -

Color Schemes Are Combinations of Colors
Color is the reflection of light off of an object into our eyes. Our eyes then read the speed of the light and tell us which color that object is. There are two major categories under the heading of color, they are: 1. Neutrals 2. Colors Neutrals are (combinations of) black and white and all grays Colors consist of: Primary colors Secondary colors Intermediate colors also known as Tertiary colors Primary Colors: are the basic colors that you cannot make by mixing. They are natural colors found in nature. They are red, yellow, and blue. Secondary Colors: are made by mixing any two secondary colors. The secondary colors are orange, violet and green. Intermediate Colors: are made by mixing a primary and a secondary color. The secondary colors are, red-violet, blue-violet, blue-green, yellow-green, yellow-orange and red-orange. Color schemes are combinations of colors. There are many different types of color combinations, however, only four of the most basic are included here. They are: • Complementary colors • Analogous colors • Warm & Cool colors • Monochromatic colors Complementary Colors: are any two colors that are opposite each other on the color wheel. Analogous Colors: are any two colors that are adjacent to (or next to) each other on the color wheel. Warm & Cool Colors: warm colors are those colors that contain combinations of red and yellow. There are six. To help you remember what a warm color is, think of the sun or fire. Cool colors are those colors that contain green and blue. There are six of these too. -

Paint Pigments— Yellow
» TECHNICAL INFORMATION ON BUILDING MATERIALS TIBM - 32 FOR UfSE IN THE DESIGN OF LOW-COST HOUSING ***** THE NATIONAL BUREAU OF STANDARDS UNITED STATES DEPARTMENT OF COMMERCE WASHINGTON, D. C. August 29, 1936 PAINT PIGMENTS— YELLOW, . BROWN, BLUE, GREEN, AND BRONZE This is urimarily^a digest of the sections of Bureau of Standards Circular No, o9> "Paint and Varnish", (November 17, 1917),'*' and Tech- nologic Paper No. 274, "Use of United States Government Suecif ication Paints and Faint Materials", (December 15, 1924), ^ Ly p, H. Walker and E. F. Hickson, dealing with general composition , characteristics, and uses of yellow, brown, blue, green, and bronze pigments. The following papers contain additional information relative to paint pigments, oil paints, and water paints: TIBM - 30 "Paint Pigments—White" TIBM - 31 "Paint Pigments—Black, Red, and Lakes" TIBM - 33 "Federal Specification . Paint Pigments and Mixing Formulas" TIM - 3U "Federal Specification Ready-Mixed Paints, Semi- paste Paints and Mixing Formulas’"' TIBM - 35 "Preparation of Paints from Paste and Dry Pigments" TIBM - 36 "Preparation of Paints from Semipaste Paints, Thinning Ready-Mixed Paints, and Preparation of Water Paints" TIBM - 43 "Aluminum Paints" Pigments are "the fine solid warticles used in the preparation of paint, and substantially insoluble in the vehicle, "3 In general, it may be ^Out of print. May be consulted in Government depositor}*- libraries. p Available from Superintendent of Documents, Government Printing Office, Washington, D. C. .(Price 10 cents). ^Qpioted from "Standard Definitions of Terms Relating to Paint 'Specifications", American Society for Testing Materials ( 1 93 3 ) ’ • • -• •• PP. 735-73 9 . 031736-C - 1 - assumed that pigments composed of very fine particles, having high re- fractive indices, provide the greatest covering power and opacity. -

Flags and Symbols � � � Gilbert Baker Designed the Rainbow flag for the 1978 San Francisco’S Gay Freedom Celebration
Flags and Symbols ! ! ! Gilbert Baker designed the rainbow flag for the 1978 San Francisco’s Gay Freedom Celebration. In the original eight-color version, pink stood for sexuality, red for life, orange for healing, yellow for the sun, green for nature, turquoise for art, indigo for harmony and violet for the soul.! " Rainbow Flag First unveiled on 12/5/98 the bisexual pride flag was designed by Michael Page. This rectangular flag consists of a broad magenta stripe at the top (representing same-gender attraction,) a broad stripe in blue at the bottoms (representing opposite- gender attractions), and a narrower deep lavender " band occupying the central fifth (which represents Bisexual Flag attraction toward both genders). The pansexual pride flag holds the colors pink, yellow and blue. The pink band symbolizes women, the blue men, and the yellow those of a non-binary gender, such as a gender bigender or gender fluid Pansexual Flag In August, 2010, after a process of getting the word out beyond the Asexual Visibility and Education Network (AVEN) and to non-English speaking areas, a flag was chosen following a vote. The black stripe represents asexuality, the grey stripe the grey-are between sexual and asexual, the white " stripe sexuality, and the purple stripe community. Asexual Flag The Transgender Pride flag was designed by Monica Helms. It was first shown at a pride parade in Phoenix, Arizona, USA in 2000. The flag represents the transgender community and consists of five horizontal stripes. Two light blue which is the traditional color for baby boys, two pink " for girls, with a white stripe in the center for those Transgender Flag who are transitioning, who feel they have a neutral gender or no gender, and those who are intersex. -

Hazard Communications (Hazcom) Symbols Nfpa
HAZARD COMMUNICATIONS (HAZCOM) SYMBOLS A training document written by: Steve Serna The Occupational Safety and Health Administration (OSHA) has determined that workers have a, “right to know” what chemical hazards are present in their particular work areas, or what chemical hazards they might encounter on their work sites. This information is written in 29 CFR 1910.1200 of the US Code. HAZCOM (Hazard Communications) relies on several written documents (MSDS & written programs) and various symbols or pictograms to inform the employee regarding chemical hazards or potential hazards. The law requires that all chemical containers/vessels have labels and adhere to a set standard; here is a quick explanation of some of the various symbols and pictograms… NFPA The National Fire Prevention Association is a private organization that catalogues and works to enact legislation for fire prevention in industrial and home settings. Most US Fire Departments rely on NFPA symbols to warn them of danger present in buildings. The NFPA Fire Diamond symbol is the common identifier along with a rating number (from 0-4) inside of a colored field to indicate a hazard rating. NFPA FIRE DIAMOND Hazcommadesimple.doc Opr: Serna Page 1 HAZARD RATINGS GUIDE For example: Diesel Fuel has an NFPA hazard rating of 0-2-0. 0 for Health (blue), 2 for Flammability (red), and 0 for Instability/Reactivity (yellow). HMIS (taken from WIKIPEDIA) The Hazardous Materials Identification System (HMIS) is a numerical hazard rating that incorporates the use of labels with color-coded bars as well as training materials. It was developed by the American Paints & Coatings Association as a compliance aid for the OSHA Hazard Communication Standard. -

R Color Cheatsheet
R Color Palettes R color cheatsheet This is for all of you who don’t know anything Finding a good color scheme for presenting data about color theory, and don’t care but want can be challenging. This color cheatsheet will help! some nice colors on your map or figure….NOW! R uses hexadecimal to represent colors TIP: When it comes to selecting a color palette, Hexadecimal is a base-16 number system used to describe DO NOT try to handpick individual colors! You will color. Red, green, and blue are each represented by two characters (#rrggbb). Each character has 16 possible waste a lot of time and the result will probably not symbols: 0,1,2,3,4,5,6,7,8,9,A,B,C,D,E,F: be all that great. R has some good packages for color palettes. Here are some of the options “00” can be interpreted as 0.0 and “FF” as 1.0 Packages: grDevices and i.e., red= #FF0000 , black=#000000, white = #FFFFFF grDevices colorRamps palettes Two additional characters (with the same scale) can be grDevices comes with the base cm.colors added to the end to describe transparency (#rrggbbaa) installation and colorRamps topo.colors terrain.colors R has 657 built in color names Example: must be installed. Each palette’s heat.colors To see a list of names: function has an argument for rainbow colors() peachpuff4 the number of colors and see P. 4 for These colors are displayed on P. 3. transparency (alpha): options R translates various color models to hex, e.g.: heat.colors(4, alpha=1) • RGB (red, green, blue): The default intensity scale in R > #FF0000FF" "#FF8000FF" "#FFFF00FF" "#FFFF80FF“ ranges from 0-1; but another commonly used scale is 0- 255. -

A New Turn Signal Ahead
At a Glance: What is the benefit of the Steady red arrow Drivers must stop flashing yellow left turn arrow? and wait. They are safer A decade-long national study Steady yellow arrow by the Federal Highway Drivers must prepare Administration found that to stop if it is safe drivers had fewer crashes to do so. with the flashing yellow arrow. They are more efficient Flashing The new signal provides more yellow arrow options for controlling traffic based Drivers must yield on variable traffic volumes since the to pedestrian and timing sequence can be made to vary oncoming traffic prior throughout the day. to making the left turn. Reminder to all drivers: Any flashing yellow signal Steady green arrow means drivers are to slow down Drivers turning left and proceed through the intersection have the right of way. with caution. A flashing red signal means motorists should come to a complete stop before proceeding. VARIATION OF FLASHING YELLOW ARROW Flashing In some instances motorists Wisconsin Department of Transportation may encounter a flashing yellow arrows yellow arrow in a three-section State Traffic Signal Systems Engineer head as seen here. The same 4802 Sheboygan Avenue, Room 501 are lighting up guidelines apply; however, no P. O. Box 7986 green arrow will be present. Madison, WI 53707-7986 all across Wisconsin (608) 261-5845 http://www.dot.wisconsin.gov/ Search for: Flashing yellow arrow 03/2011 How the new four-head NEW FOUR-HEAD SIGNAL FLASHING YELLOW ARROWS A SAFER YELLOW TURN SIGNAL signals work: A new style traffic signal is being As usual, red means stop. -

Fruit and Vegetable Color Chart Red Orange/ Yellow Green Blue
Fruit and Vegetable Color Chart Color Why It’s Good For You Fruit and Veggie Examples • Lycopene: • Reduces risk of prostate cancer tomatoes, watermelon, red cabbage, red • Reduces risk of hypertension bell peppers • Decreases LDL (bad) cholesterol levels • Quercetin • Decreases plaque formation apples, cherries, cranberries, red onions, Red • Reduces risk of lung and breast cancers beets • Improves aerobic endurance capacity • Anthocyanins • Reduces risk of heart disease red raspberries, sweet cherries, • Improves brain function and memory strawberries, cranberries, beets, red apples, • Improves balance kidney beans • Improves vision • Beta Carotene/Vitamin A Carrots, pumpkins, sweet potatoes, • Improves eye health cantaloupes, apricots, peaches, papaya, Reduces risk of cancer and heart disease Orange / • grapefruits, persimmons, butternut squash • Helps to fight infection Yellow • Bioflavonoids oranges, grapefruit, lemons, tangerines, • Reduces risk of heart disease clementines, peaches, papaya, apricots, • Improves brain function nectarines, pineapple • Beta Carotene/Vitamin A kale, spinach, lettuce, mustard greens, • Keeps eyes healthy cabbage, swiss chard, collard greens, • Reduces risk of cancer and heart disease parsley, basil, beet greens, endive, chives, • Helps to fight infections arugula, asparagus • Folate • Reduces risk of birth defects spinach, endive, lettuce, asparagus, • Protection against neurodegenerative mustard greens, green beans, collard disorders greens, okra, broccoli Green • Helps to fight infections • Regulates -

Yellow Wild Indigo July 2017
Wildflower Spot – July 2017 John Clayton Chapter of the Virginia Native Plant Society - Baptisia tinctoria tolerate drought and poor soils. Found in ev ery county in Virginia, the range extends from Past-president of the John Clayton southern Maine to Georgia and Tennessee. YellowChapter, VNPS Wild Indigo By Helen Hamilton, The showy flowers attract butterflies and other- pollinators, and the leaves host the caterpillars of the Wild Indigo Duskywing and Silver-spot Bright yellow pea-shaped flowers cover this ted Skipper. The foliage is pest resistant, and shrubby wildflower from April through August. distasteful to deer and rabbits. Yellow Wild The mound of foliage grows 2-3 feet tall on Indigo is a member of the Pea Family, and as many-branched stems carrying silvery green a legume, the roots contain bacteria that fix 3-parted leaves. After- nitrogen, enriching the the flowers fade they soils where they grow. are replaced by in This is a good choice - flated seedpods that for a wildlife or cottage are attractive in indoor garden or a dry mead arrangements. ow. The yellow flowers look wonderful whenAscle - Yellow Wild Indigo is piasplanted tuberosa with orange - easy to grow in average, Butterfly Weed ( well-drained soil, in ), and pur full sun or light shade.- ple and white asters. Young plants may not flower vigorous- Native Americans used ly since they expend the plant for various much energy devel medicinal purposes; oping strong taproots. studies have shown During the third year the extract stimulates the upper half of the wasthe immune used by system.early plant will produce more Yellow Wild Indigo - blossoms.