Wilkinson's Catalyst
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organometallic Chemistry BASIC PRINCIPLES, APPLICATIONS, and a FEW CASE STUDIES
Safety Moment TYLER LAB GROUP MEETING 1 Safety Moment TYLER LAB GROUP MEETING 2 Metal Hydrides: Benchtop vs. Box Hydride = :H- Hydrides are powerful Lewis bases and reducing agent ◦ Exothermically form H2 (this should scare you) ◦ Heating leads to faster reactivity ◦ H evolution leads to rapid increase in pressure2 ◦ Uncontrolled reactions easily cause runaway exotherm, class D fire, explosion, and death/unemployment LiAlH is the #1 chemical cause of fatality in chemical4 industry 3 Metal Hydrides: “I want to commit the murder I was imprisoned for†.” LiAlH4 ◦ Insanely irritating (serious safety hazard) ◦ Extremely moisture sensitive (don’t leave out for >2 minutes) ◦ Ethereal mixtures are pyrophoric! DiBuAl-H ◦ Pyrophoric – it will explode upon exposure to oxygen NaEt3BH ◦ Pyrophoric in solution LiH and NaH ◦ Can be handled on the benchtop (not >2 minutes) ◦ Parrafin oil dispersions much safer KH ◦ Pyrophoric if not in a dispersion ◦ Handle with extreme care! † Sirius Black, Harry Potter and the Prisoner of Azkaban 4 Metal Hydrides: “I want to commit the murder I was imprisoned for†.” CaH2 ◦ Very safe to handle on the benchtop Pt-H, Pd-H, Ni-H ◦ All very pyrophoric NaBH4 ◦ Very safe in general Other hydrides ◦ Treat as pyrophoric ◦ Transition metal hydrides vary in hydridic strength ◦ General rule of thumb: if it does hydrogenations, it is probably pyrophoric ◦ If they’re in organics of any kind, they are probably pyrophoric † Sirius Black, Harry Potter and the Prisoner of Azkaban 5 Organometallic Chemistry BASIC PRINCIPLES, APPLICATIONS, -

Recent Advances on Mechanistic Studies on C–H Activation
Open Chem., 2018; 16: 1001–1058 Review Article Open Access Daniel Gallego*, Edwin A. Baquero Recent Advances on Mechanistic Studies on C–H Activation Catalyzed by Base Metals https:// doi.org/10.1515/chem-2018-0102 received March 26, 2018; accepted June 3, 2018. 1Introduction Abstract: During the last ten years, base metals have Application in organic synthesis of transition metal- become very attractive to the organometallic and catalytic catalyzed cross coupling reactions has been positioned community on activation of C-H bonds for their catalytic as one of the most important breakthroughs during the functionalization. In contrast to the statement that new millennia. The seminal works based on Pd–catalysts base metals differ on their mode of action most of the in the 70’s by Heck, Noyori and Suzuki set a new frontier manuscripts mistakenly rely on well-studied mechanisms between homogeneous catalysis and synthetic organic for precious metals while proposing plausible chemistry [1-5]. Late transition metals, mostly the precious mechanisms. Consequently, few literature examples metals, stand as the most versatile catalytic systems for a are found where a thorough mechanistic investigation variety of functionalization reactions demonstrating their have been conducted with strong support either by robustness in several applications in organic synthesis [6- theoretical calculations or experimentation. Therefore, 12]. Owing to the common interest in the catalysts mode we consider of highly scientific interest reviewing the of action by many research groups, nowadays we have a last advances on mechanistic studies on Fe, Co and Mn wide understanding of the mechanistic aspects of precious on C-H functionalization in order to get a deep insight on metal-catalyzed reactions. -

2.1 the 18-ELECTRON RULE the 18E Rule1 Is a Way to Help Us Decide
30 GENERAL PROPERTIES OF ORGANOMETALLIC COMPLEXES 2.1 THE 18-ELECTRON RULE The 18e rule1 is a way to help us decide whether a given d-block transition metal organometallic complex is likely to be stable. Not all the organic formulas we can write down correspond to stable species. For example, CH5 requires a 5-valent carbon and is therefore not stable. Stable compounds, such as CH4,have the noble gas octet, and so carbon can be thought of as following an 8e rule. This corresponds to carbon using its s and three p orbitals to form four filled bonding orbitals and four unfilled antibonding orbitals. On the covalent model, we can consider that of the eight electrons required to fill the bonding orbitals, four come from carbon and one each comes from the four H substituents. We can therefore think of each H atom as being a 1e ligand to carbon. To assign a formal oxidation state to carbon in an organic molecule, we impose an ionic model by artificially dissecting it into ions. Each electron pair in any bond is assigned to the most electronegative of the two atoms or groups that constitute the bond. For methane, this dissection gives C4− + 4H+, with carbon as the more electronegative element. This makes methane an 8e compound with an oxidation state of −4, usually written C(-IV). Note that the net electron count always remains the same, whether we adopt the covalent (4e {Catom}+4 × 1e {4H atoms}=8e) or ionic (8e{C4−ion}+4 × 0e{4H+ions}=8e) model. -

Assessing the Feasibility of a One-Pot, Tandem Olefin Metathesis and Isomerization Sequence to Synthesize Conjugated Aromatic Olefins
University of Tennessee at Chattanooga UTC Scholar Student Research, Creative Works, and Honors Theses Publications 8-2016 Assessing the feasibility of a one-pot, tandem olefin metathesis and isomerization sequence to synthesize conjugated aromatic olefins Ajay D. Makwana University of Tennessee at Chattanooga, [email protected] Follow this and additional works at: https://scholar.utc.edu/honors-theses Part of the Chemistry Commons Recommended Citation Makwana, Ajay D., "Assessing the feasibility of a one-pot, tandem olefin metathesis and isomerization sequence to synthesize conjugated aromatic olefins" (2016). Honors Theses. This Theses is brought to you for free and open access by the Student Research, Creative Works, and Publications at UTC Scholar. It has been accepted for inclusion in Honors Theses by an authorized administrator of UTC Scholar. For more information, please contact [email protected]. Assessing the Feasibility of a One-Pot, Tandem Olefin Metathesis and Isomerization Sequence to Synthesize Conjugated Aromatic Olefins Ajay D. Makwana Departmental Honors Thesis The University of Tennessee at Chattanooga Department of Chemistry Project Director: Dr. Kyle S. Knight Examination Date: April 4, 2016 Members of Examination Committee: Dr. John P. Lee Dr. Han J. Park Dr. David Giles 1 Abstract The synthesis of substituted phenylpropene dimers using a one-pot, tandem olefin metathesis and isomerization sequence has been studied. This sequence relies on the facilitated, in-situ conversion of a ruthenium carbene species (Ru=C) to a ruthenium hydride species (Ru-H) upon addition of an inorganic hydride source. Three separate reactions occur within one reaction flask: 1) olefin metathesis of the starting phenylpropene to yield phenylpropene dimer via Ru=C catalyst, 2) conversion of Ru=C to Ru-H via addition of an inorganic hydride source, 3) isomerization of phenylpropene dimer via insertion and β-hydride elimination to yield conjugated product. -

Transmetallation Versus Hydride Elimination
DOI: 10.1002/chem.201102678 Transmetallation Versus b-Hydride Elimination: The Role of 1,4- Benzoquinone in Chelation-Controlled Arylation Reactions with Arylboronic Acids Christian Skçld,*[a] Jonatan Kleimark,[b] Alejandro Trejos,[a] Luke R. Odell,[a] Sten O. Nilsson Lill,[b] Per-Ola Norrby,[b] and Mats Larhed[a] Abstract: The formation of an atypical, um, which is necessary to complete the complex. The association of BQ lowers saturated, diarylated, Heck/Suzuki, catalytic cycle, this electron-deficient the free-energy barrier for transmetal- domino product produced under oxida- alkene opens up a low-energy reaction lation of the s-alkyl complex to create tive Heck reaction conditions, employ- pathway from the post-insertion s-alkyl a pathway that is energetically lower ing arylboronic acids and a chelating than the oxidative Heck reaction path- vinyl ether, has been investigated by way. Furthermore, the calculations Keywords: arylation · CÀC cou- DFT calculations. The calculations showed that the reaction is made pling · density functional calcula- highlight the crucial role of 1,4-benzo- viable by BQ-mediated reductive elimi- tions · elimination · palladium · quinone (BQ) in the reaction. In addi- nation and leads to the saturated di- transmetallation tion to its role as an oxidant of palladi- arylatedACHTUNGRE product. Introduction step of the reaction, it was initially performed by using stoi- ACHTUNGRE chiometric amounts of Pd(OAc)2. More recent develop- The Mizoroki–Heck reaction[1] is a convenient and versatile ments have rendered the reaction catalytic by the re-oxida- method for the formation of carbon–carbon bonds through tion of Pd0 to PdII and today the oxidative Heck reaction is the vinylation or arylation of alkenes.[2] The reaction is per- an established and useful variant of the vinylic substitution formed by Pd0 catalysis in which the active PdII–vinyl or reaction.[4] –aryl intermediate is produced by oxidative addition of aryl One strategy to achieve regio- and stereoselectivity in the halides or pseudo-halides. -
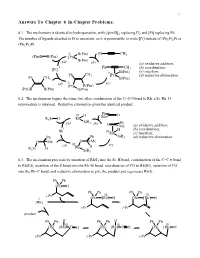
Answers to Chapter 6 In-Chapter Problems
1 Answers To Chapter 6 In-Chapter Problems. 6.1. The mechanism is identical to hydrogenation, with [(pin)B]2 replacing H2 and [Pt] replacing Pd. The number of ligands attached to Pt is uncertain, so it is permissible to write [Pt] instead of (Ph3P)2Pt or (Ph3P)3Pt. II B(Pin) Ph CH3 (Pin)B B(Pin) [Pt] B(Pin) (a) (b) (a) oxidative addition; 0 Ph CH (b) coordination; [Pt] 3 II B(Pin) (c) insertion; Ph CH3 [Pt] (d) reductive elimination. Ph CH3 B(Pin) (d) II (c) [Pt] B(Pin) (Pin)B B(Pin) B(Pin) 6.2. The mechanism begins the same, but after coordination of the C=O π bond to Rh, a Si–Rh–O intermediate is obtained. Reductive elimination gives the identical product. Ph III H Me O R3Si H Rh (a) SiR3 (b) Ph O Me (a) oxidative addition; I H (b) coordination; Rh IIIRh (c) insertion; Ph Ph SiR3 (d) reductive elimination. O Me O Me III (c) (d) Rh H R3Si H SiR3 6.3. The mechanism proceeds by insertion of Rh(I) into the Si–H bond, coordination of the C=C π bond to Rh(III), insertion of the π bond into the Rh–Si bond, coordination of CO to Rh(III), insertion of CO into the Rh–C bond, and reductive elimination to give the product and regenerate Rh(I). Ph Ph OSi H Ph Ph Ph Ph i-Pr III III I OSi [Rh] H OSi [Rh] H [Rh] i-Pr i-Pr product Ph Ph H Ph Ph H Ph Ph III III III OSi [Rh] C O OSi [Rh] C O OSi [Rh] H i-Pr i-Pr i-Pr Chapter 6 2 6.4. -
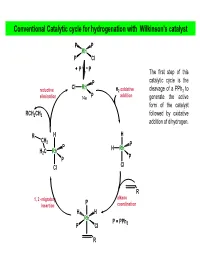
Conventional Catalytic Cycle for Hydrogenation with Wilkinson's Catalyst
Conventional Catalytic cycle for hydrogenation with Wilkinson’s catalyst P P Rh P Cl PP The first step of this P catalytic cycle is the Cl Rh reductive H2 oxidative cleavage of a PPh3 to elimination 14e P addition generate the active form of the catalyst RCH2CH3 followed by oxidative addition of dihydrogen. R H H CH 2 P P H Rh H2C Rh P P Cl Cl R alkene 1, 2 -migratory P insertion coordination H H Rh P = PPh P Cl 3 R AJELIAS L7-S18 catalytic cycle for hydrogenation H H2 oxidative P P addition H P Kinetic studies have Rh Rh shown that the P Cl P Cl dissociation of PPh3 P from the distorted P square planar complex PP (due to trans RhCl(PPh3)3 in effect of H ) H benzene occurs only to a very small extent H2 oxidative P (k = 2.3 × 10–7 M at P addition HRh Cl Rh 25°C), and P P under an atmosphere Cl of H2, a solution of RCH2CH3 RhCl(PPh3)3 becomes reductive alkene yellow as a result of elimination the oxidative addition of H2 to give cis- R H P CH2 H2RhCl(PPh3)3. P H H H2CRh Rh P 1, 2 -migratory P Cl insertion Cl R The trans effect is the labilization (making unstable) of ligands that are trans to certain other ligands, which can thus be regarded as trans-directing ligands. The intensity of the trans effect (as measured by the increase in rate of substitution of the trans ligand) follows this sequence: H2O, OH− < NH3 < py < Cl− < Br− < I−, < PR3, CH3− < H−, NO, CO AJELIAS L7-S19 Relative reactivity of alkenes for homogenous catalytic hydrogenation • Cis alkenes undergo hydrogenation more readily than trans alkenes •Internal and branched alkenes -

Basics of Catalysis and Kinetics
Basics of Catalysis and Kinetics Nobel laureates in catalysis: Haber (1918) Ziegler and Natta (1963) Wilkinson, Fischer (1973) Knowles, Noyori, Sharpless (2001) Grubbs, Schrock, Chauvin (2006) Ertl (2007) Heck, Negishi, Suzuki (2010) G. Poli Definition The concept of catalyst was first introduced by Berzelius in the 19th century. Subsequently, Ostwald came up with the definition that we still use today: “A catalyst is a substance that increases the rate of a chemical reaction, without being consumed or produced”… Berzelius, J. J. Ann. Chim. Phys. 1836, 61, 146-151 Ostwald, W. Z. Phys. Chem. 1894, 15, 705-706 G. Poli The Catalyst and the Rate of a Reaction ΔG non catalyzed catalyzed A B Reaction coordinate The catalyst changes only the rate of the reaction, and does not change the thermodynamics. However, the temperature can affect equilibrium concentrations: ΔG = ΔH - T ΔS G. Poli Historical basis for the Analysis of Catalytic Kinetics The Michaelis-Menten Kinetics G. Poli Thermal, Catalyzed, Inhibited, and Promoted Reactions I II III IV uncatalyzed catalysis inhibition stoichimetric process (no catalysis) promotion (no catalysis) A A A A Acat Bcat Ainhib Binhib Aprom B B (+ cat) B Bprom SM is stabilized SM is stabilized more than TS --> a further reaction is less than TS the uncatalyzed path needed to liberate B is preferred G. Poli Thermal, Catalyzed, Inhibited, and Promoted Reactions O NH HN CCl3 Ar O CCl3 xylenes 140°C type I Ar CCl3 CCl3 O NH PdCl2(PhCN)2 (5 mol%) O NH type II THF, rt N H H N B N N N N B Pt N N 1. -

Decomposition of Ruthenium Olefin Metathesis Catalyst
catalysts Review Decomposition of Ruthenium OlefinOlefin Metathesis CatalystMetathesis Catalyst Magdalena Jawiczuk 1,, Anna Anna Marczyk Marczyk 1,21,2 andand Bartosz Bartosz Trzaskowski Trzaskowski 1,* 1,* 1 1 CentreCentre of of New New Technologies, Technologies, University University of of Warsaw, Warsaw, Banacha Banacha 2c, 2c, 02-097 02-097 Warsaw, Warsaw, Poland; [email protected]@cent.uw.edu.pl (M.J.); (M.J.); [email protected] [email protected] (A.M.) (A.M.) 2 Faculty of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland 2 Faculty of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland * Correspondence: [email protected] * Correspondence: [email protected] Received: 28 28 June 2020; Accepted: 02 2 AugustAugust 2020;2020; Published:Published: 5date August 2020 Abstract: RutheniumRuthenium olefin olefin metathesis metathesis catalysts catalysts are are one one of of the most commonly used class of catalysts. There There are are multiple multiple reviews reviews on on their their us useses in in various branches of chemistry and other sciences but a detailed review of their decomposition is missing, despite a large number of recent and important advances advances in in this this field. field. In In particular, particular, in in the the last last five five years years several several new new mechanism mechanism of decomposition,of decomposition, both both olefin-driven olefin-driven as well as well as induc as induceded by external by external agents, agents, have have been been suggested suggested and usedand usedto explain to explain differences differences in the decomposition in the decomposition rates and rates the metathesis and the metathesis activities activitiesof both standard, of both N-heterocyclicstandard, N-heterocyclic carbene-based carbene-based systems and systems the recently and the developed recently developed cyclic alkyl cyclic amino alkyl carbene- amino containingcarbene-containing complexes. -

Chapter 1 the Basic Chemistry of Organopalladium Compounds
Chapter 1 The Basic Chemistry of Organopalladium Compounds 1.1 Characteristic Features of Pd-Promoted or -Catalyzed Reactions There are several features which make reactions involving Pd catalysts and reagents particularly useful and versatile among many transition metals used for organic synthesis. Most importantly, Pd catalysts offer an abundance of possibilities of carbon–carbon bond formation. Importance of the carbon–carbon bond forma- tion in organic synthesis needs no explanation. No other transition metals can offer such versatile methods of the carbon–carbon bond formations as Pd. Tol- erance of Pd catalysts and reagents to many functional groups such as carbonyl and hydroxy groups is the second important feature. Pd-catalyzed reactions can be carried out without protection of these functional groups. Although reactions involving Pd should be carried out carefully, Pd reagents and catalysts are not very sensitive to oxygen and moisture, and even to acid in many reactions catalyzed by Pd–phosphine complexes. It is enough to apply precautions to avoid oxidation of coordinated phosphines, and this can be done easily. On the other hand, the Ni(0) complex is extremely sensitive to oxygen. Of course, Pd is a noble metal and expensive. Its price frequently fluctuates drastically. A few years ago, Pd was more expensive than Pt and Au but cheaper than Rh. As of October 2003, the comparative prices of the noble metals were: Pd (1), Au (1.8), Rh (2.8), Pt (3.3), Ru (0.2). Recently the price of Pd has dropped dramatically, and Pt is currently the most expensive noble metal. Also, the toxicity of Pd has posed no serious problem so far. -

The Emergence of Transition Metal-Mediated Hydrothiolation of Unsaturated Carbon-Carbon Bonds: a Mechanistic Outlook Ricardo Castarlenas,* Andrea Di Giuseppe, Jesús J
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Digital.CSIC Hydrothiolation of Unsaturated Bonds The Emergence of Transition Metal-Mediated Hydrothiolation of Unsaturated Carbon-Carbon Bonds: A Mechanistic Outlook Ricardo Castarlenas,* Andrea Di Giuseppe, Jesús J. Pérez-Torrente, Luis A. Oro* The hydrothiolation of unsaturated carbon-carbon bonds is a practical and atom-economical approach for the incorporation of sulfur into organic frameworks. In recent years, we have witnessed the development of a range of transition metal-based catalytic systems for the control of the regio- and stereoselectivity. This minireview aims to highlight the mechanistic background behind this transformation in order to help for the design of more specific and active organometallic hydrothiolation catalysts. 1. Introduction in-depth understanding of the mechanistic issues, and indeed, it could be useful to other diverse hydroelementation transformations The development of efficient synthetic methods for the such as hydroalkoxylation, hydrophosphination, hydroamination, or incorporation of sulfur into organic frameworks is nowadays an hydroacylation, among others. Our aim in this concise survey is to important task due to the practical applications of this type of analyze the different mechanistic pathways rather than to present a compounds as pharmaceuticals, functional materials, or synthetic comprehensive overview of a research area that has already been reagents.[1-4] In this context, one of the simplest and atom- covered in several recent reviews.[1,5] The sulfa-michael additions economical approach is the direct addition of sulfur and hydrogen mediated by organocatalysts remains beyond the scope of this atoms from thiols to unsaturated carbon-carbon bonds, commonly minireview. -

Insertion Reactions • Oxidative Addition and Substitution Allow Us to Assemble 1E and 2E Ligands on the Metal, Respectively
Insertion Reactions • Oxidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. • With insertion, and its reverse reaction, elimination, we can now combine and transform these ligands within the coordination sphere, and ultimately expel these transformed ligands to form free organic compounds. • There are two main types of insertion 1) 1,1 insertion in which the metal and the X ligand end up bound to the same (,)(1,1) atom 2) 1,2 insertion in which the metal and the X ligand end up bound to adjacent (1,2) atoms of an L‐type ligand. • The type of insertion observed in any given case depends on the nature of the 2e inserting ligand. • For example: CO gives only 1,1 insertion ethylene gives only 1,2 insertion, in which the M and the X end up on adjacent atoms of what was the 2e X‐type ligand. In general, η1 ligands tend to give 1,1 insertion and η2 ligands give 1,2 insertion • SO2 is the only common ligan d tha t can give bthboth types of itiinsertion; as a ligan d, SO2 can be η1 (S) or η2 (S, O). • In principle, insertion reactions are reversible, but just as we saw for oxidative addition and reductive elimination previously, for many ligands only one of the two possible directions is observed in practice, probably because this direction is strongly favored thermodynamically. •A2e vacant site is generated by 1,1 and 1,2 insertion reactions. • Thissite can be occupidied byanextlternal 2e ligan d and the itiinsertion prodtduct tdtrapped.