Original Articles Sustained Activation of Insulin Receptors Internalized in GLUT4 Vesicles of Insulin-Stimulated Skeletal Muscle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antibody-Dependent Cellular Cytotoxicity in HIV Infection
CE: Namrta; QAD/AIDS-D-18-00733; Total nos of Pages: 13; AIDS-D-18-00733 EDITORIAL REVIEW Antibody-dependent cellular cytotoxicity in HIV infection Donald N. Forthala,b and Andres Finzic,d Interactions between the Fc segment of IgG and its receptors (FcgRs) found on cells such as natural killer cells, monocytes, macrophages and neutrophils can potentially mediate antiviral effects in the setting of HIV and related infections. We review the potential role of Fc-FcR interactions in HIV, SIV and SHIV infections, with an emphasis on antibody- dependent cellular cytotoxicity (ADCC). Notably, these viruses employ various strate- gies, including CD4 down-regulation and BST-2/tetherin antagonism to limit the effect of ADCC. Although correlative data suggest that ADCC participates in both protection and control of established infection, there is little direct evidence in support of either role. Direct evidence does, however, implicate an FcgR-dependent function in aug- menting the beneficial in-vivo activity of neutralizing antibodies. Copyright ß 2018 Wolters Kluwer Health, Inc. All rights reserved. AIDS 2018, 32:000–000 Keywords: antibody-dependent cellular cytotoxicity, CD4, Fc receptor, HIV, natural killer cell, phagocytosis, simian immunodeficiency virus, simian/human immunodeficiency virus Introduction antibody-dependent enhancement, the interested reader is directed elsewhere [1,2]. In addition, detailed Much of the antiviral activity of antibody is mediated by treatments of FcR biology can be found in recent interactions between the Fc segment of immunoglobulin reviews [3,4]. and Fc receptors (FcRs) present on many different cell types. Such interactions could have a beneficial impact on ADCC occurs when antibody forms a bridge between a viral infection through, for example, antibody-dependent target cell bearing foreign antigens on its surface and an cellular cytotoxicity (ADCC), phagocytosis, or trogocy- effector cell, typically a natural killer cell expressing FcRs. -

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

List of Genes Used in Cell Type Enrichment Analysis
List of genes used in cell type enrichment analysis Metagene Cell type Immunity ADAM28 Activated B cell Adaptive CD180 Activated B cell Adaptive CD79B Activated B cell Adaptive BLK Activated B cell Adaptive CD19 Activated B cell Adaptive MS4A1 Activated B cell Adaptive TNFRSF17 Activated B cell Adaptive IGHM Activated B cell Adaptive GNG7 Activated B cell Adaptive MICAL3 Activated B cell Adaptive SPIB Activated B cell Adaptive HLA-DOB Activated B cell Adaptive IGKC Activated B cell Adaptive PNOC Activated B cell Adaptive FCRL2 Activated B cell Adaptive BACH2 Activated B cell Adaptive CR2 Activated B cell Adaptive TCL1A Activated B cell Adaptive AKNA Activated B cell Adaptive ARHGAP25 Activated B cell Adaptive CCL21 Activated B cell Adaptive CD27 Activated B cell Adaptive CD38 Activated B cell Adaptive CLEC17A Activated B cell Adaptive CLEC9A Activated B cell Adaptive CLECL1 Activated B cell Adaptive AIM2 Activated CD4 T cell Adaptive BIRC3 Activated CD4 T cell Adaptive BRIP1 Activated CD4 T cell Adaptive CCL20 Activated CD4 T cell Adaptive CCL4 Activated CD4 T cell Adaptive CCL5 Activated CD4 T cell Adaptive CCNB1 Activated CD4 T cell Adaptive CCR7 Activated CD4 T cell Adaptive DUSP2 Activated CD4 T cell Adaptive ESCO2 Activated CD4 T cell Adaptive ETS1 Activated CD4 T cell Adaptive EXO1 Activated CD4 T cell Adaptive EXOC6 Activated CD4 T cell Adaptive IARS Activated CD4 T cell Adaptive ITK Activated CD4 T cell Adaptive KIF11 Activated CD4 T cell Adaptive KNTC1 Activated CD4 T cell Adaptive NUF2 Activated CD4 T cell Adaptive PRC1 Activated -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

ISOTYPE-SPECIFIC IMMUNOREGULATION Iga-Binding Factors Produced by Fca Receptor-Positive T Cell Hybridomas Regulate Iga Responses
ISOTYPE-SPECIFIC IMMUNOREGULATION IgA-binding Factors Produced by Fca Receptor-positive T Cell Hybridomas Regulate IgA Responses BY HIROSHI KIYONO,* LISA M. MOSTELLER-BARNUM, ANNETTE M. PITTS, SHANE I. WILLIAMSON, SUZANNE M. MICHALEK ANn JERRY R. McGHEE From the *Department of Oral Biology and Preventive Dentistry, the Department of Microbiology, the Institute of Dental Research, and the Comprehensive Cancer Center, University of Alabama at Birmingham, University Station, Birmingham, Alabama 35294 Fc receptors (FcR) 1, membrane glycoproteins which bind Ig molecules via their Fc region, have been identified (1) on the surface of various cell types. For example, FcR for IgG are expressed on macrophages, B and T lymphocytes, cytotoxic T lymphocytes, and natural killer cells. FcR are important in immune regulation, and several studies have addressed their role in isotype-specific responses. In this regard, T lymphocyte subpopulations have been described with FcR for IgM (FcttR) (2), XgD (Fc6R) (3), IgG (Fc~,R) (4), IgE (Fc~R) (5), and IgA (FcaR) (6). Previous studies (1, 7, 8) have shown that FcyR-bearing (Fc-yR +) T cells and T cell hybridomas regulate IgG production. These T cells release Fc~,R molecules, termed IgG-binding factors (IBF~) that suppress IgG synthesis (7, 8). Others (9-12) have demonstrated that IgE-binding factors (IBFe) released from FceR + T lymphocytes regulate IgE immune responses. IBFt possess opposite regulatory functions; one factor specifically enhances the IgE response, while a second factor suppresses this isotype (10, 11). Both IgE-potentiating factor and IgE-suppressive factor are produced by the same FctR + T cell subpopulation (9, 12). -

Arming Tumor-Associated Macrophages to Reverse Epithelial
Published OnlineFirst August 15, 2019; DOI: 10.1158/0008-5472.CAN-19-1246 Cancer Tumor Biology and Immunology Research Arming Tumor-Associated Macrophages to Reverse Epithelial Cancer Progression Hiromi I.Wettersten1,2,3, Sara M.Weis1,2,3, Paulina Pathria1,2,Tami Von Schalscha1,2,3, Toshiyuki Minami1,2,3, Judith A. Varner1,2, and David A. Cheresh1,2,3 Abstract Tumor-associated macrophages (TAM) are highly expressed it engaged macrophages but not natural killer (NK) cells to within the tumor microenvironment of a wide range of cancers, induce antibody-dependent cellular cytotoxicity (ADCC) of where they exert a protumor phenotype by promoting tumor avb3-expressing tumor cells despite their expression of the cell growth and suppressing antitumor immune function. CD47 "don't eat me" signal. In contrast to strategies designed Here, we show that TAM accumulation in human and mouse to eliminate TAMs, these findings suggest that anti-avb3 tumors correlates with tumor cell expression of integrin avb3, represents a promising immunotherapeutic approach to redi- a known driver of epithelial cancer progression and drug rect TAMs to serve as tumor killers for late-stage or drug- resistance. A monoclonal antibody targeting avb3 (LM609) resistant cancers. exploited the coenrichment of avb3 and TAMs to not only eradicate highly aggressive drug-resistant human lung and Significance: Therapeutic antibodies are commonly engi- pancreas cancers in mice, but also to prevent the emergence neered to optimize engagement of NK cells as effectors. In of circulating tumor cells. Importantly, this antitumor activity contrast, LM609 targets avb3 to suppress tumor progression in mice was eliminated following macrophage depletion. -

FCRL4 Is an Fc Receptor for Systemic Iga, but Not Mucosal Secretory Iga Yanling Liu, Sofiya Goroshko, Leslie Y
FCRL4 Is an Fc Receptor for Systemic IgA, but Not Mucosal Secretory IgA Yanling Liu, Sofiya Goroshko, Leslie Y. T. Leung, Shilan Dong, Srijit Khan, Paolo Campisi, Evan J. Propst, Nikolaus This information is current as E. Wolter, Eyal Grunebaum and Götz R. A. Ehrhardt of September 28, 2021. J Immunol published online 8 June 2020 http://www.jimmunol.org/content/early/2020/06/05/jimmun ol.2000293 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2020/06/05/jimmunol.200029 Material 3.DCSupplemental http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2020 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published June 8, 2020, doi:10.4049/jimmunol.2000293 The Journal of Immunology FCRL4 Is an Fc Receptor for Systemic IgA, but Not Mucosal Secretory IgA Yanling Liu,* Sofiya Goroshko,* Leslie Y. T. Leung,* Shilan Dong,* Srijit Khan,* Paolo Campisi,† Evan J. -

Mouse and Human Fcr Effector Functions
Pierre Bruhns Mouse and human FcR effector € Friederike Jonsson functions Authors’ addresses Summary: Mouse and human FcRs have been a major focus of Pierre Bruhns1,2, Friederike J€onsson1,2 attention not only of the scientific community, through the cloning 1Unite des Anticorps en Therapie et Pathologie, and characterization of novel receptors, and of the medical commu- Departement d’Immunologie, Institut Pasteur, Paris, nity, through the identification of polymorphisms and linkage to France. disease but also of the pharmaceutical community, through the iden- 2INSERM, U760, Paris, France. tification of FcRs as targets for therapy or engineering of Fc domains for the generation of enhanced therapeutic antibodies. The Correspondence to: availability of knockout mouse lines for every single mouse FcR, of Pierre Bruhns multiple or cell-specific—‘a la carte’—FcR knockouts and the Unite des Anticorps en Therapie et Pathologie increasing generation of hFcR transgenics enable powerful in vivo Departement d’Immunologie approaches for the study of mouse and human FcR biology. Institut Pasteur This review will present the landscape of the current FcR family, 25 rue du Docteur Roux their effector functions and the in vivo models at hand to study 75015 Paris, France them. These in vivo models were recently instrumental in re-defining Tel.: +33145688629 the properties and effector functions of FcRs that had been over- e-mail: [email protected] looked or discarded from previous analyses. A particular focus will be made on the (mis)concepts on the role of high-affinity Acknowledgements IgG receptors in vivo and on results from antibody engineering We thank our colleagues for advice: Ulrich Blank & Renato to enhance or abrogate antibody effector functions mediated by Monteiro (FacultedeMedecine Site X. -

Engineered Type 1 Regulatory T Cells Designed for Clinical Use Kill Primary
ARTICLE Acute Myeloid Leukemia Engineered type 1 regulatory T cells designed Ferrata Storti Foundation for clinical use kill primary pediatric acute myeloid leukemia cells Brandon Cieniewicz,1* Molly Javier Uyeda,1,2* Ping (Pauline) Chen,1 Ece Canan Sayitoglu,1 Jeffrey Mao-Hwa Liu,1 Grazia Andolfi,3 Katharine Greenthal,1 Alice Bertaina,1,4 Silvia Gregori,3 Rosa Bacchetta,1,4 Norman James Lacayo,1 Alma-Martina Cepika1,4# and Maria Grazia Roncarolo1,2,4# Haematologica 2021 Volume 106(10):2588-2597 1Department of Pediatrics, Division of Stem Cell Transplantation and Regenerative Medicine, Stanford School of Medicine, Stanford, CA, USA; 2Stanford Institute for Stem Cell Biology and Regenerative Medicine, Stanford School of Medicine, Stanford, CA, USA; 3San Raffaele Telethon Institute for Gene Therapy, Milan, Italy and 4Center for Definitive and Curative Medicine, Stanford School of Medicine, Stanford, CA, USA *BC and MJU contributed equally as co-first authors #AMC and MGR contributed equally as co-senior authors ABSTRACT ype 1 regulatory (Tr1) T cells induced by enforced expression of interleukin-10 (LV-10) are being developed as a novel treatment for Tchemotherapy-resistant myeloid leukemias. In vivo, LV-10 cells do not cause graft-versus-host disease while mediating graft-versus-leukemia effect against adult acute myeloid leukemia (AML). Since pediatric AML (pAML) and adult AML are different on a genetic and epigenetic level, we investigate herein whether LV-10 cells also efficiently kill pAML cells. We show that the majority of primary pAML are killed by LV-10 cells, with different levels of sensitivity to killing. Transcriptionally, pAML sensitive to LV-10 killing expressed a myeloid maturation signature. -

Human FCAR/CD89 Fluorescein-Conjugated Antibody
Human FCAR/CD89 Fluorescein-conjugated Antibody Monoclonal Mouse IgG1 Clone # 488032 Catalog Number: FAB3939F 100 Tests DESCRIPTION Species Reactivity Human Specificity Detects human FCAR/CD89 in direct ELISAs. In direct ELISAs, no crossreactivity with Fcγ RIA, RIIA, or RIIIB is observed. Source Monoclonal Mouse IgG1 Clone # 488032 Purification Protein A or G purified from hybridoma culture supernatant Immunogen NS0 mouse myeloma cell line transfected with human FCAR/CD89 Gln22Lys287 Accession # P24071 Conjugate Fluorescein Excitation Wavelength: 488 nm Emission Wavelength: 515545 nm (FITC) Formulation Supplied in a saline solution containing BSA and Sodium Azide. See Certificate of Analysis for details. *Contains <0.1% Sodium Azide, which is not hazardous at this concentration according to GHS classifications. Refer to the Safety Data Sheet (SDS) for additional information and handling instructions. APPLICATIONS Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website. Recommended Sample Concentration Flow Cytometry 10 µL/106 cells See Below DATA Flow Cytometry Detection of FCAR/CD89 in Human Blood Granulocytes by Flow Cytometry. Human peripheral blood granulocytes were stained with Mouse AntiHuman FCAR/CD89 Fluoresceinconjugated Monoclonal Antibody (Catalog # FAB3939F, filled histogram) or isotype control antibody (Catalog # IC002F, open histogram). View our protocol for Staining Membraneassociated Proteins. PREPARATION AND STORAGE Shipping The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. Stability & Storage Protect from light. Do not freeze. l 12 months from date of receipt, 2 to 8 °C as supplied. -
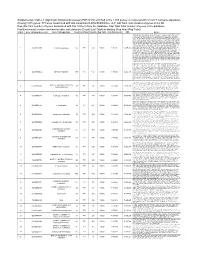
(FDR<0.05) Enriched in the 1015 Genes Co-Clustered with Known T
Supplementary Table 3. Significant biological processes (FDR<0.05) enriched in the 1,015 genes co-clustered with known T cell gene signatures. Among 1,015 genes, 771 were associated with GO annotation in DAVID database v6.7. List Total: total number of genes in my list. Pop Hits: total number of genes associated with this GO term from the database. Pop Total: total number of genes in the database. Fold Enrichment: relative enrichment ratio, calculated by (Count)/(List Total) divided by (Pop Hits)/(Pop Total). Index Gene Ontology Accession Gene Ontology Name Count List Total Pop Hits Pop Total Fold Enrichment FDR Genes AQP9, C1QC, B2M, LILRA1, LILRA2, CLEC4E, S1PR4, LILRA4, IFNG, LILRA6, CLEC4A, VNN1, ERAP2, FAS, CRTAM, C5AR1, GBP5, NCF2, NCF1, NCF4, SERPING1, HLA-DQA2, HLA-DQA1, PDCD1LG2, LILRB1, CCR9, C1QA, C1QB, LILRB2, CCR7, CCR6, UNC13D, CCR5, CD40LG, CCR4, LILRB3, CCR2, LILRB4, HLA-DPA1, VSIG4, HLA-DRA, IL1R2, IL1R1, HLA-DRB1, OAS3, ACP5, OAS1, OAS2, CD74, IFI35, ZAP70, FCER1G, HLA-DRB5, HLA-DPB1, HLA-DOA, HLA-DOB, DHX58, BLNK, IL23R, KIR2DS4, CD300C, SLAMF7, OASL, RGS1, APOL1, CD300A, HMHB1, CD209, CLEC7A, LY86, LY9, CLNK, FCRL4, SH2D1A, NOD2, HAMP, CCL3L1, CCL3L3, TICAM2, ICAM1, GZMA, CMKLR1, LY96, WAS, IL18BP, LAX1, TNFSF12- TNFSF13, HLA-DQB1, CSF2, GPR183, CCR1, GPR65, CXCL9, NCF1C, IL7R, CLEC10A, CCL24, CCL22, CYP27B1, CCL23, FCGR1C, FTHL3, FCGR1A, FCGR1B, BCL3, C2, CD27, CD28, FYB, IL18R1, IL7, CD1C, CTLA4, CCL19, CD1B, CD1A, TRIM22, CD180, CD1E, CCL18, CCL17, CCL13, FCGR2B, FCGR2C, P2RY14, LIME1, CD14, IL16, IL18, TLR1, TNFSF15, -

Definition of Immunoglobulin a Receptors on Eosinophils and Their Enhanced Expression in Allergic Individuals
Definition of immunoglobulin A receptors on eosinophils and their enhanced expression in allergic individuals. R C Monteiro, … , G L Gartland, H Kubagawa J Clin Invest. 1993;92(4):1681-1685. https://doi.org/10.1172/JCI116754. Research Article Fc alpha receptors (Fc alpha R), detected by the binding of IgA and by anti-Fc alpha R antibodies, were found to be differentially expressed on eosinophils and neutrophils. Neutrophils were the major granulocyte population expressing Fc alpha R, and they expressed much higher levels of Fc alpha R than eosinophils. The expression of Fc alpha R by eosinophils could be upregulated approximately threefold by Ca2+ ionophore treatment in a dose- and time-dependent manner. This effect, which was blocked by a chelating agent, was not duplicated by other cellular stimuli. Eosinophils in allergic individuals displayed enhanced Fc alpha R expression, whereas neutrophils did not. The Fc alpha R on eosinophils had a higher molecular mass (70-100 kD) than those identified on neutrophils (55-75 kD). However, removal of N-linked carbohydrates from Fc alpha R of eosinophils and neutrophils revealed a major protein core of 32 kD for both cell types. The data indicate that expression of Fc alpha R molecules with a characteristic glycosylation pattern is upregulated on eosinophils in allergic individuals. Find the latest version: https://jci.me/116754/pdf Definition of Immunoglobulin A Receptors on Eosinophils and Their Enhanced Expression in Allergic Individuals Renato C. Monteiro, * Robert W. Hostoffer, Max D. Cooper,* James R. Bonner, G. Larry Gartland, and Hiromi Kubagawa Division of Developmental and Clinical Immunology, Departments of Pediatrics, Medicine, Pathology, and Microbiology, and the Comprehensive Cancer Center, University ofAlabama at Birmingham; and the *Howard Hughes Medical Institute, Birmingham, Alabama 35294 Abstract binding to specific Fc7yR and FcER present on the cell surface (3, 4).