External Cephalic Version (Ecv) Author: Scope
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Diagnostic Impact of Limited, Screening Obstetric Ultrasound When Performed by Midwives in Rural Uganda
Journal of Perinatology (2014) 34, 508–512 © 2014 Nature America, Inc. All rights reserved 0743-8346/14 www.nature.com/jp ORIGINAL ARTICLE The diagnostic impact of limited, screening obstetric ultrasound when performed by midwives in rural Uganda JO Swanson1, MG Kawooya2, DL Swanson1, DS Hippe1, P Dungu-Matovu2 and R Nathan1 OBJECTIVE: To evaluate the diagnostic impact of limited obstetric ultrasound (US) in identifying high-risk pregnancies when used as a screening tool by midwives in rural Uganda. STUDY DESIGN: This was an institutional review board-approved prospective study of expecting mothers in rural Uganda who underwent clinical and US exams as part of their standard antenatal care visit in a local health center in the Isingiro district of Uganda. The midwives documented clinical impressions before performing a limited obstetric US on the same patient. The clinical findings were then compared with the subsequent US findings to determine the diagnostic impact. The midwives were US-naive before participating in the 6-week training course for limited obstetric US. RESULT: Midwife-performed screening obstetric US altered the clinical diagnosis in up to 12% clinical encounters. This diagnostic impact is less (6.7 to 7.4%) if the early third trimester diagnosis of malpresentation is excluded. The quality assurance review of midwives’ imaging demonstrated 100% sensitivity and specificity in the diagnosing gestational number, and 90% sensitivity and 96% specificity in the diagnosis of fetal presentation. CONCLUSION: Limited, screening obstetric US performed by midwives with focused, obstetric US training demonstrates the diagnostic impact for identifying conditions associated with high-risk pregnancies in 6.7 to 12% of patients screened. -

Breech Presentation at Delivery: a Marker for Congenital Anomaly?
Journal of Perinatology (2014) 34, 11–15 & 2014 Nature America, Inc. All rights reserved 0743-8346/14 www.nature.com/jp ORIGINAL ARTICLE Breech presentation at delivery: a marker for congenital anomaly? D Mostello1, JJ Chang2, F Bai2, J Wang3, C Guild4, K Stamps2 and TL Leet2,{ OBJECTIVE: To determine whether congenital anomalies are associated with breech presentation at the time of birth. STUDY DESIGN: A population-based, retrospective cohort study was conducted among 460 147 women with singleton live births using the Missouri Birth Defects Registry, which includes all defects diagnosed during the first year of life. Maternal and obstetric characteristics and outcomes between breech and cephalic presentation groups were compared using w2-square statistic and Student’s t-test. Multivariable binary logistic regression analysis was used to estimate adjusted odds ratios (aORs) and 95% confidence intervals (CIs). RESULT: At least one congenital anomaly was more likely present among infants breech at birth (11.7%) than in those with cephalic presentation (5.1%), whether full-term (9.4 vs 4.6%) or preterm (20.1 vs 11.6%). The relationship between breech presentation and congenital anomaly was stronger among full-term births (aOR 2.09, CI 1.96, 2.23, term vs 1.40, CI 1.26, 1.55, preterm), but not in all categories of anomalies. CONCLUSION: Breech presentation at delivery is a marker for the presence of congenital anomaly. Infants delivered breech deserve special scrutiny for the presence of malformation. Journal of Perinatology (2014) 34, 11–15; -

A Guide to Obstetrical Coding Production of This Document Is Made Possible by Financial Contributions from Health Canada and Provincial and Territorial Governments
ICD-10-CA | CCI A Guide to Obstetrical Coding Production of this document is made possible by financial contributions from Health Canada and provincial and territorial governments. The views expressed herein do not necessarily represent the views of Health Canada or any provincial or territorial government. Unless otherwise indicated, this product uses data provided by Canada’s provinces and territories. All rights reserved. The contents of this publication may be reproduced unaltered, in whole or in part and by any means, solely for non-commercial purposes, provided that the Canadian Institute for Health Information is properly and fully acknowledged as the copyright owner. Any reproduction or use of this publication or its contents for any commercial purpose requires the prior written authorization of the Canadian Institute for Health Information. Reproduction or use that suggests endorsement by, or affiliation with, the Canadian Institute for Health Information is prohibited. For permission or information, please contact CIHI: Canadian Institute for Health Information 495 Richmond Road, Suite 600 Ottawa, Ontario K2A 4H6 Phone: 613-241-7860 Fax: 613-241-8120 www.cihi.ca [email protected] © 2018 Canadian Institute for Health Information Cette publication est aussi disponible en français sous le titre Guide de codification des données en obstétrique. Table of contents About CIHI ................................................................................................................................. 6 Chapter 1: Introduction .............................................................................................................. -

Twin Pregnancy with Triploidy and Co-Existing Live Twin Progressing to Viability: Case Study and Literature Review
Obstetrics & Gynecology International Journal Case Report Open Access Twin pregnancy with triploidy and co-existing live twin progressing to viability: Case study and literature review Abstract Volume 9 Issue 4 - 2018 Background: Twin pregnancies consisting of a triploid fetus with molar change and a 1,2 1,2 1,2 healthy coexisting fetus is an extremely rare phenomenon. Triploidy rarely advances to the Cingiloglu P, Yeung T, Sekar R second trimester, most resulting in a miscarriage, termination or stillbirth. Only five cases 1Royal Brisbane and Women’s Hospital, Women’s and Newborn of a triploid fetus and a healthy co-twin have been reported in literature. We present the first Services, Australia documented case of a live delivery of both triploid fetus and coexisting viable twin. 2University of Queensland, Australia Case presentation: A 23-year old woman, gravida 2, para 1 was admitted at 26+4 weeks Correspondence: Pinar Cingiloglu, Women’s and Newborn gestation with DCDA twins and a shortened cervix. Sonography revealed polyhydramnios, Services, Royal Brisbane and Women’s Hospital, Bowen Bridge an abnormal cystic placenta, and small aortic valve for the presenting twin. Emergency Road&, Butterfield St, Herston Queensland Australia, Tel +61 caesarean section was performed at 28 weeks gestation for cord prolapse. Twin A was born (07) 3646 8111, Email [email protected] a live infant with ambiguous genitalia, syndactyly, epicanthic folds and cerebral cysts. Twin B was born a healthy live male. Histopathology revealed features consistent with Received: June 13, 2018 | Published: July 13, 2018 partial hydatidiform mole, and cytogenetics revealed a diandric triploidy in twin A. -

Gtg-No-20B-Breech-Presentation.Pdf
Guideline No. 20b December 2006 THE MANAGEMENT OF BREECH PRESENTATION This is the third edition of the guideline originally published in 1999 and revised in 2001 under the same title. 1. Purpose and scope The aim of this guideline is to provide up-to-date information on methods of delivery for women with breech presentation. The scope is confined to decision making regarding the route of delivery and choice of various techniques used during delivery. It does not include antenatal or postnatal care. External cephalic version is the topic of a separate RCOG Green-top Guideline No. 20a: ECV and Reducing the Incidence of Breech Presentation. 2. Background The incidence of breech presentation decreases from about 20% at 28 weeks of gestation to 3–4% at term, as most babies turn spontaneously to the cephalic presentation. This appears to be an active process whereby a normally formed and active baby adopts the position of ‘best fit’ in a normal intrauterine space. Persistent breech presentation may be associated with abnormalities of the baby, the amniotic fluid volume, the placental localisation or the uterus. It may be due to an otherwise insignificant factor such as cornual placental position or it may apparently be due to chance. There is higher perinatal mortality and morbidity with breech than cephalic presentation, due principally to prematurity, congenital malformations and birth asphyxia or trauma.1,2 Caesarean section for breech presentation has been suggested as a way of reducing the associated perinatal problems2,3 and in many countries in Northern Europe and North America caesarean section has become the normal mode of breech delivery. -

Advising Women with Diabetes in Pregnancy to Express Breastmilk In
Articles Advising women with diabetes in pregnancy to express breastmilk in late pregnancy (Diabetes and Antenatal Milk Expressing [DAME]): a multicentre, unblinded, randomised controlled trial Della A Forster, Anita M Moorhead, Susan E Jacobs, Peter G Davis, Susan P Walker, Kerri M McEgan, Gillian F Opie, Susan M Donath, Lisa Gold, Catharine McNamara, Amanda Aylward, Christine East, Rachael Ford, Lisa H Amir Summary Lancet 2017; 389: 2204–13 Background Infants of women with diabetes in pregnancy are at increased risk of hypoglycaemia, admission to a See Editorial page 2163 neonatal intensive care unit (NICU), and not being exclusively breastfed. Many clinicians encourage women with See Comment page 2167 diabetes in pregnancy to express and store breastmilk in late pregnancy, yet no evidence exists for this practice. We Judith Lumley Centre, School of aimed to determine the safety and efficacy of antenatal expressing in women with diabetes in pregnancy. Nursing and Midwifery, La Trobe University, Methods We did a multicentre, two-group, unblinded, randomised controlled trial in six hospitals in Victoria, Australia. Melbourne, VIC, Australia (Prof D A Forster PhD, We recruited women with pre-existing or gestational diabetes in a singleton pregnancy from 34 to 37 weeks’ gestation A M Moorhead RM, and randomly assigned them (1:1) to either expressing breastmilk twice per day from 36 weeks’ gestation (antenatal L H Amir PhD); Royal Women’s expressing) or standard care (usual midwifery and obstetric care, supplemented by support from a diabetes educator). Hospital, Parkville, VIC, Randomisation was done with a computerised random number generator in blocks of size two and four, and was Australia (Prof D A Forster, A M Moorhead, S E Jacobs MD, stratified by site, parity, and diabetes type. -

Obstetrics Seventh Edition
PROLOGObstetrics seventh edition The American College of Obstetricians and Gynecologists WOMEN’S HEALTH CARE PHYSICIANS Obstetrics seventh edition Assessment Book The American College of Obstetricians and Gynecologists WOMEN’S HEALTH CARE PHYSICIANS ISBN 978-1-934984-22-2 Copyright 2013 by the American College of Obstetricians and Gynecologists. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, posted on the Internet, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the publisher. 12345/76543 The American College of Obstetricians and Gynecologists 409 12th Street, SW PO Box 96920 Washington, DC 20090-6920 Contributors PROLOG Editorial and Advisory Committee CHAIR MEMBERS Ronald T. Burkman Jr, MD Bernard Gonik, MD Professor of Obstetrics and Professor and Fann Srere Chair of Gynecology Perinatal Medicine Tufts University School of Medicine Division of Maternal–Fetal Medicine Division of General Obstetrics and Department of Obstetrics and Gynecology Gynecology Department of Obstetrics and Wayne State University School of Gynecology Medicine Baystate Medical Center Detroit, Michigan Springfield, Massachusetts Louis Weinstein, MD Past Paul A. and Eloise B. Bowers Professor and Chair Department of Obstetrics and Gynecology Thomas Jefferson University Philadelphia, Pennsylvania Linda Van Le, MD Leonard Palumbo Distinguished Professor UNC Gynecologic Oncology University of North Carolina School of Medicine Chapel Hill, North Carolina PROLOG Task Force for Obstetrics, Seventh Edition COCHAIRS MEMBERS Vincenzo Berghella, MD Cynthia Chazotte, MD Director, Division of Maternal–Fetal Professor of Clinical Obstetrics and Medicine Gynecology and Women’s Health Professor, Department of Obstetrics Department of Obstetrics and Gynecology and Gynecology Weiler Hospital of the Albert Einstein Thomas Jefferson University College of Medicine Philadelphia, Pennsylvania Bronx, New York George A. -

Pretest Obstetrics and Gynecology
Obstetrics and Gynecology PreTestTM Self-Assessment and Review Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. The authors and the publisher of this work have checked with sources believed to be reliable in their efforts to provide information that is complete and generally in accord with the standards accepted at the time of publication. However, in view of the possibility of human error or changes in medical sciences, neither the authors nor the publisher nor any other party who has been involved in the preparation or publication of this work warrants that the information contained herein is in every respect accurate or complete, and they disclaim all responsibility for any errors or omissions or for the results obtained from use of the information contained in this work. Readers are encouraged to confirm the information contained herein with other sources. For example and in particular, readers are advised to check the prod- uct information sheet included in the package of each drug they plan to administer to be certain that the information contained in this work is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. This recommendation is of particular importance in connection with new or infrequently used drugs. Obstetrics and Gynecology PreTestTM Self-Assessment and Review Twelfth Edition Karen M. Schneider, MD Associate Professor Department of Obstetrics, Gynecology, and Reproductive Sciences University of Texas Houston Medical School Houston, Texas Stephen K. Patrick, MD Residency Program Director Obstetrics and Gynecology The Methodist Health System Dallas Dallas, Texas New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto Copyright © 2009 by The McGraw-Hill Companies, Inc. -
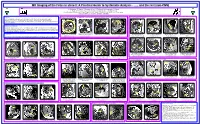
MR Imaging of the Fetus in Utero II: a Practical Guide to Systematic Analysis - …
MR Imaging of the Fetus in utero II: A Practical Guide to Systematic Analysis - ….. and the rest (non-CNS) A Robinson, S Blaser, S Pantazi, A Toi, D Chitayat, M Gundogan, G Ryan The Hospital for Sick Children Departments of Diagnostic Imaging and Clinical & Metabolic Genetics Mount Sinai Hospital Departments of Medical Imaging, Prenatal Diagnosis & Medical Genetics and Obstetrics & Gynaecology The University of Toronto, Ontario, Canada Purpose Biometry (see CNS poster) Check situs – work out which side is fetal left/right BEFORE checking internal organs Check the kidneys & bladder Most of our examinations are performed for CNS indications, with which most neuroradiologists should feel comfortable. However these examinations also include a variable amount of non-CNS anatomy with which neuroradiologists might feel less comfortable, but which should be reviewed routinely also, and not simply glossed over or even worse ignored. Materials and Methods Retrospective analysis of was performed in over 190 consecutive fetal MR examinations performed for CNS and non- CNS indications. Analysis included, but was not limited to, evaluation of thoracic and abdominal situs, lung parenchyma, diaphragms, liver and gallbladder, stomach, kidneys (including biometry), bladder, cord insertion, cord vessels, placental site and morphology, and amniotic fluid volume. Abdominal circumference Normal situs Normal levocardia Normal aortic arch Normal kidneys - axial Multicystic dysplastic kidneys Normal kidneys - sagittal Horseshoe kidney Conclusion Correct level -

Physiological and Pathological Control of Fetal Heart Rate Patterns
Cardiotocography – Physiological and Pathological Control of Fetal Heart Rate Patterns Terence Lao Department of Obstetrics & Gynaecology, Queen Mary Hospital, The University of Hong Kong Cardiotocography • A continuous recording of the fetal heart rate and uterine activities by electronic means • Limitations in its usefulness is related to – Problems with the monitors – Lack of understanding of pathophysiology – Carelessness / laziness in the attendants Basic Understanding of the Fetal Heart Rate Patterns • Represents fetal physiology • Different patterns represent the different results of the interaction of many factors • Fetal compromise only diagnosed in association with clinical assessment and interpreted with respect to the probability of fetal compromise Fetal Response to Hypoxia (1) Hypoxia ←↓Blood Flow ↓↓ ↓ PO2 ↑PCO2 ↓↓ Metabolic acidosis ← Respiratory acidosis ⇓ Redistribution of blood flow to vital organs Bradycardia, and slightly ↓cardiac output ↓oxygen consumption Fetal Response to Hypoxia (2) ↓↓ FHR variability FHR ↓ variability, retained / ↑ rate decelerations ⇓⇓ Compensated State Decompensated State (Normal cortical functions (Decrease cerebral cerebral oxygenation oxygenation, eventual maintained, can last up cellular damage to 60 min in experimental animals) Umbilical blood flow and hypoxia • Acute moderate hypoxia → no effect • Severe moderate hypoxia →↓due to myocardial depression, catecholamines What is Fetal Distress (1) ? = Hypoxaemia (low PO2) ? = Acidaemia (low pH) ? = General depression (low Apgar Score) But- -

The Physiology of the Uterus in Labor
The Physiology of the Uterus in Labor D. N. DANFORTH, M.D., R. J. GRAHAM, B.M. and A. C. IVY, M.D.l HE purpose of this article is to uterine horns (8). This ring becomes T synthesize into a rather complete fairly evident during labor at the junc picture the experimental observations tion of the upper and lower segments. that have been made during the past It is a more or less definite, tapering ten years in our laboratory regarding ledge, the formation of which is due the processes concerned in the evacu to the greater thickness or "retraction" ation of the uterus. of the muscle fibers of the upper than the lower segment. (This r ing is the The Anatomic and Physiologic " fundal or cornal sphincter" in the Divisions of the Uterus dog.) In obstructed labors it becomes It is important first to obtain a clear a very pronounced ring or band and concept (a) of the anatomical divisions is then called Bandl's ring. The ap of the human uterus, which may be pearance of a Bandl's ring means -- traced directly to those in lower forms, threatened rupture of the uterus in the and (b) of the obstetrical or physio lower segment ; it is a pathological re logical divisions. See figure 1. traction ring. This is a subject that has been greatly III. The lower uterine segment is an confused because our knowledge of the alogous to the isthmus uteri oj Asch gross and microscopic anatomy of the off in the non-pregnant uterus. In human uterus, and of the embryology, woman its upper level is generally comparative anatomy, and physiology marked by the reflection of the per of the uterus has developed in ~ hap itoneum. -

Ophthalmic Complications of Amniocentesis
Eye (1990) 4, 845-849 Ophthalmic Complications of Amniocentesis G. NAYLOR, J. P. ROPER, H. E. WILLS HAW Birmingham Summary Reports of ocular damage from amniocentesis needles are rare. We report four cases of ocular perforation in which an amniocentesis needle may have been the cause and a proven case of central nervous system perforation during amniocentesis which resulted in hemianopia and gaze palsy. Ocular damage during amniocentesis may be commoner than the paucity of reports would suggest, and should be considered in all cases of congenital ocular conditions. Amniocentesis was introduced in 1930' as part which to perform the amniocentesis. The of the technique of amniography to determine increased risks of the procedure were placental position in cases of painless bleeding explained to the mother who elected to pro in the third trimester of pregnancy. Since then ceed. Amniocentesis proved difficult, only the technique has been mainly used in the 2ml of blood stained liquor being aspirated management of haemolytic disease· of new following two needle insertions. Subsequent born, assessment of fetal maturity, and pre analysis of the fluid revealed an alphafetopro natal genetic diagnosis. tein (AFP ) of 240mg/1 (normal 36mg/l at 16 Most amniocenteses now take place in mid weeks gestation). Acetylcholinesterase trimester, and although some studies point to (ACH) assay showed two bands to be present. a slightly increased risk of fetal loss,2,3 the The blood in the sample was 100% fetal. NICHD group in 19764 concluded that amnio Cytogenetic results of the cultured cells centesis was a 'highly accurate and safe pro showed a normal male karyotype-46XY.