The Usage of Coal-Layered Double Oxide for Removal of Toxic Dye From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phenolphthalein, 0.5% in 50% Ethanol Safety Data Sheet According to Federal Register / Vol
Phenolphthalein, 0.5% in 50% Ethanol Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Issue date: 12/17/2013 Revision date: 07/21/2020 Supersedes: 01/23/2018 Version: 1.5 SECTION 1: Identification 1.1. Identification Product form : Mixtures Product name : Phenolphthalein, 0.5% in 50% Ethanol Product code : LC18200 1.2. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only. Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem, Inc. 1010 Jackson's Pointe Ct. Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or +1-703-741-5970 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS US classification Flammable liquids Category 3 H226 Flammable liquid and vapor Carcinogenicity Category 1A H350 May cause cancer Reproductive toxicity Category 2 H361 Suspected of damaging the unborn child. (oral) Specific target organ toxicity (single exposure) Category 1 H370 Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS US labeling Hazard pictograms (GHS US) : Signal word (GHS US) : Danger Hazard statements (GHS US) : H226 - Flammable liquid and vapor H350 - May cause cancer H361 - Suspected of damaging the unborn child. (oral) H370 - Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) Precautionary statements (GHS US) : P201 - Obtain special instructions before use. -

The Action of the Phosphatases of Human Brain on Lipid Phosphate Esters by K
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.19.1.12 on 1 February 1956. Downloaded from J. Neurol. Neurosurg. Psychiat., 1956, 19, 12 THE ACTION OF THE PHOSPHATASES OF HUMAN BRAIN ON LIPID PHOSPHATE ESTERS BY K. P. STRICKLAND*, R. H. S. THOMPSON, and G. R. WEBSTER From the Department of Chemical Pathology, Guy's Hospital Medical School, London, Much work, using both histochemical and therefore to study the action of the phosphatases in standard biochemical techniques, has been carried human brain on the " lipid phosphate esters out on the phosphatases of peripheral nerve. It is i.e., on the various monophosphate esters that occur known that this tissue contains both alkaline in the sphingomyelins, cephalins, and lecithins. In (Landow, Kabat, and Newman, 1942) and acid addition to ox- and 3-glycerophosphate we have phosphatases (Wolf, Kabat, and Newman, 1943), therefore used phosphoryl choline, phosphoryl and the changes in the levels of these enzymes in ethanolamine, phosphoryl serine, and inositol nerves undergoing Wallerian degeneration following monophosphate as substrates for the phospho- transection have been studied by several groups of monoesterases, and have measured their rates guest. Protected by copyright. of investigators (see Hollinger, Rossiter, and Upmalis, hydrolysis by brain preparations over the pH range 1952). 4*5 to 100. Phosphatase activity in brain was first demon- Plimmer and Burch (1937) had earlier reported strated by Kay (1928), and in 1934 Edlbacher, that phosphoryl choline and phosphoryl ethanol- Goldschmidt, and Schiiippi, using ox brain, showed amine are hydrolysed by the phosphatases of bone, that both acid and alkaline phosphatases are kidney, and intestine, but thepH at which the hydro- present in this tissue. -

Student Safety Sheets Dyes, Stains & Indicators
Student safety sheets 70 Dyes, stains & indicators Substance Hazard Comment Solid dyes, stains & indicators including: DANGER: May include one or more of the following Acridine orange, Congo Red (Direct dye 28), Crystal violet statements: fatal/toxic if swallowed/in contact (methyl violet, Gentian Violet, Gram’s stain), Ethidium TOXIC HEALTH with skin/ if inhaled; causes severe skin burns & bromide, Malachite green (solvent green 1), Methyl eye damage/ serious eye damage; may cause orange, Nigrosin, Phenolphthalein, Rosaniline, Safranin allergy or asthma symptoms or breathing CORR. IRRIT. difficulties if inhaled; may cause genetic defects/ cancer/damage fertility or the unborn child; causes damages to organs/through prolonged or ENVIRONMENT repeated exposure. Solid dyes, stains & indicators including Alizarin (1,2- WARNING: May include one or more of the dihydroxyanthraquinone), Alizarin Red S, Aluminon (tri- following statements: harmful if swallowed/in ammonium aurine tricarboxylate), Aniline Blue (cotton / contact with skin/if inhaled; causes skin/serious spirit blue), Brilliant yellow, Cresol Red, DCPIP (2,6-dichl- eye irritation; may cause allergic skin reaction; orophenolindophenol, phenolindo-2,6-dichlorophenol, HEALTH suspected of causing genetic PIDCP), Direct Red 23, Disperse Yellow 7, Dithizone (di- defects/cancer/damaging fertility or the unborn phenylthiocarbazone), Eosin (Eosin Y), Eriochrome Black T child; may cause damage to organs/respiratory (Solochrome black), Fluorescein (& disodium salt), Haem- HARMFUL irritation/drowsiness or dizziness/damage to atoxylin, HHSNNA (Patton & Reeder’s indicator), Indigo, organs through prolonged or repeated exposure. Magenta (basic Fuchsin), May-Grunwald stain, Methyl- ene blue, Methyl green, Orcein, Phenol Red, Procion ENVIRON. dyes, Pyronin, Resazurin, Sudan I/II/IV dyes, Sudan black (Solvent Black 3), Thymol blue, Xylene cyanol FF Solid dyes, stains & indicators including Some dyes may contain hazardous impurities and Acid blue 40, Blue dextran, Bromocresol green, many have not been well researched. -

Phenolphthalein Is Pink in Base Acid–Base Indicators SCIENTIFIC
Phenolphthalein Is Pink in Base Acid–Base Indicators SCIENTIFIC Introduction Phenolphthalein is a large organic molecule used as an acid–base indicator. Phenolphthalein turns a bright red color as its solution becomes basic. In a strongly basic solution, this red color fades to colorless. Concepts • Acid–base indicators • Chemical equilibrium Background Phenolphthalein has the colorless structure shown in Figure 1 when the solution pH <8. As the solution becomes basic and the pH increases (pH 8–10), the phenolphthalein molecule (abbreviated H2P) loses two hydrogen ions to form the red- violet dianion (abbreviated P2–) shown in Figure 2. At a high pH, the P2– ions reacts with hydroxide ions to form the colorless POH3– ion. HO – – O –O O O OH O O– C C C – – + OH C O CO– 2 CO2 OH – CO2 O P2– Red 2– POH3– Colorless3– Figure 1. H2P is colorless. Figure 2. P is red. Figure 3. POH is colorless. 2– The colorless-to-red transition of H2P to P (Equation 1) is very rapid and the red color develops instantly when the pH reaches its transition range (pH 8–10). If the concentration of hydroxide ions remains high, the red P2– dianion will slowly combine with hydroxide ions to form a third species, POH3– (Equation 2), which is again colorless. The rate of this second reaction is much slower than the first and depends on the concentration of phenolphthalein and hydroxide ions. Thus, the color of the red P2– species will gradually fade in a basic solution. fast 2– + H2P → P + 2H Equation 1 Colorless Red slow P2– + OH– → POH3– Equation 2 Red Colorless Materials (for each demonstration) Hydrochloric acid solution, HCl, 3 M, 10 mL Beral-type pipet, disposable Phenolphthalein solution, 0.5%, 3mL Test tubes, borosilicate, 16 5 100mm, 3 Sodium hydroxide pellets, NaOH, 2 Test tube rack Sodium hydroxide solution, NaOH, 3 M, 5 mL Wash bottle Safety Precautions Hydrochloric acid solution is toxic and corrosive to eyes and skin tissue. -

MATERIAL SAFETY DATA SHEET Phenolphthalein
MATERIAL SAFETY DATA SHEET Phenolphthalein Section 1 Chemical Product and Company Identification MSDS Name: Phenolphthalein Catalog 147710000, 147711000, 147715000, 417180000, 417180025, 417181000, 41718 Numbers: 5000 Synonyms: Company Identification: Acros Organics BVBA Janssen Pharmaceuticalaan 3a 2440 Geel, Belgium Company Identification: (USA) Acros Organics One Reagent Lane Fair Lawn, NJ 07410 For information in the US, call: 800ACROS01 For information in Europe, call: +32 14 57 52 11 Emergency Number, Europe: +32 14 57 52 99 Emergency Number US: 2017967100 CHEMTREC Phone Number, US: 8004249300 CHEMTREC Phone Number, Europe: 7035273887 Section 2 Composition, Information on Ingredients CAS# Chemical Name: % EINECS# 77098 Phenolphthalein 2010047 Hazard Symbols: XN Risk Phrases: 22 40 Section 3 Hazards Identification EMERGENCY OVERVIEW Harmful if swallowed. Limited evidence of a carcinogenic effect. Potential Health Effects Eye: May cause eye irritation. Skin: May cause skin irritation. May be harmful if absorbed through the skin. Ingestion: Harmful if swallowed. May cause irritation of the digestive tract. Ingestion may cause fever, blood pressure increase and other unspecified vascular effects. Inhalation: May cause respiratory tract irritation. May be harmful if inhaled. Chronic: Limited evidence of a carcinogenic effect. Section 4 First Aid Measures Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Ingestion: Get medical aid. Wash mouth out with water. -
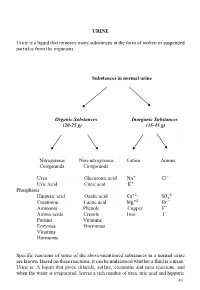
URINE Urine Is a Liquid That Removes Many Substances in the Form Of
URINE Urine is a liquid that removes many substances in the form of molten or suspended particles from the organism. Substances in normal urine Organic Substances Inorganic Substances (20-25 g) (35-45 g) Nitrogenous Non-nitrogenous Cation Anions Compounds Compounds Urea Glucuronic acid Na+ CI− Uric Acid Citric acid K+ Phosphates +2 −2 Hippuric acid Oxalic acid Ca SO4 Creatinine Lactic acid Mg+2 Br− Ammonia Phenols Copper F− Amino acids Cresols Iron I− Purines Vitamins Enzymes Hormones Vitamins Hormones Specific reactions of some of the above-mentioned substances in a normal urine are known. Based on these reactions, it can be understood whether a fluid is a urine. Urine is: A liquid that gives chloride, sulfate, creatinine and urea reactions, and when the water is evaporated, leaves a rich residue of urea, uric acid and hippuric 49 acid. Understanding whether a liquid is urine or not: •Determination of chloride, sulfate, urea, creatinine in liquid •Search for urea in the residual after the liquid has been evaporated Experiments will be carried out by sampling 2-3 ml into the tube. 1-Determination of chloride Liquid + a few drops of concentrated HNO3+ 2-3 drops of 5% AgNO3→ AgCl ↓ (white sediment) Nitric acid (HNO3 prevents the precipitation of phosphate and carbonate which could be precipitated by silver nitrate (AgNO3). 2-Determination of sulphate Liquid + several drops of 10% HCl + 2-3 drops BaCl2→ BaSO4↓ (white sediment) 3-Determination of urea Searched by two experiments: • Sodium hypobromite (NaOBr) • Using the enzyme urease a- Sodium -

Flavonols and Antioxidant Activity of Physalis Peruviana L. Fruit at Two Maturity Stages
Acta Scientiarum http://www.uem.br/acta ISSN printed: 1806-2563 ISSN on-line: 1807-8664 Doi: 10.4025/actascitechnol.v35i2.13265 Flavonols and antioxidant activity of Physalis peruviana L. fruit at two maturity stages Silvana Licodiedoff1*, Luciano André Deitos Koslowski2 and Rosemary Hoffmann Ribani1 1Programa de Pós-graduação em Tecnologia de Alimentos, Centro Politécnico, Universidade Federal do Paraná, Rua Francisco H. dos Santos, s/n, 81531-980, Cx. Postal 19011, Curitiba, Paraná, Brazil. 2Departamento de Engenharia Química, Universidade da Região de Joinville, Joinville, Santa Catarina, Brazil. *Author for correspondence. E-mail: [email protected] ABSTRACT. Since the characteristics of the fresh fruit of cape gooseberry (Physalis peruviana L.) are little known, its valorization and use are impaired. The fruit’s bioactive compounds at two stages of maturity, start and end of maturity, are evaluated, with differentiating colors between green-yellow and orange for two sizes of the fruit. The ratio between sugars and acids increased from the beginning to the end of maturity. Quercetin was not found in the samples. Nevertheless, rutin was predominant in small and large size mature sample, followed by greenish yellow (start of maturity) color of the small size fruit, with values ranging between 6.904 and 6.761 μg g-1 and 5.891 to 4.465 μg g-1, respectively. Myricetin rates ranged between 1.085 and 1.170 μg g-1 and 1.110 to 1.309 μg g-1 for greenish yellow and orange fruits, respectively. These results characterize the fruit of Physalis peruviana L. as a source of phenolic compounds in food. -

Phenolphthalein, 1% in 95% Ethanol Safety Data Sheet According to Federal Register / Vol
Phenolphthalein, 1% in 95% Ethanol Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 12/17/2013 Revision date: 01/23/2018 Supersedes: 06/13/2014 Version: 2.1 SECTION 1: Identification 1.1. Identification Product form : Mixtures Product name : Phenolphthalein, 1% in 95% Ethanol Product code : LC18220 1.2. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only. Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Flammable liquids H225 Highly flammable liquid and vapour Category 2 Acute toxicity (oral) H302 Harmful if swallowed Category 4 Skin corrosion/irritation H315 Causes skin irritation Category 2 Serious eye damage/eye H319 Causes serious eye irritation irritation Category 2A Carcinogenicity Category 2 H351 Suspected of causing cancer (oral) Reproductive toxicity H361 Suspected of damaging fertility or the unborn child (oral) Category 2 Specific target organ H370 Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) toxicity (single exposure) Category 1 Specific target organ H335 May cause respiratory irritation toxicity (single exposure) Category 3 Full text of H statements : see section 16 2.2. -

We Have Little Information Regarding the Nucleic Acid of Bacteria, Whether Concerning a Pathogenic Or a Non-Pathogenic Bacteria
The Journal of Biochemistry, Vol. 28, No. 3. ON THE NUCLEIC ACID OF TYPHOID BACILLUS OF MICE. BY SYUZO AKASI. (From the Medico-chemical Institute, Kyoto Imperial University. Director: Prof. K. Mayeda.) (Received for publication, June 20, 1938) We have little information regarding the nucleic acid of bacteria, whether concerning a pathogenic or a non-pathogenic bacteria. Studies of nucleic acids have been made with coli bacillus by Schaffer, Forkoff, and Jone (1922), with azotobacter by Mockeridge (1924), with tubercle bacillus by Ruppel (1898-99) and later by Johnson and Brown (1922 & 1923), and with timothy bacillus (1931) as well as diphtheria bacillus (1932) re cently by Coghil1. All the workers were truly in accord with the finding that bacterial cells contain generally a nucleic acid as a cell constituent, but the individual results indicated a marked difference from each other, so that as to the question whether it deals with the animal or the plant type of nucleic acid, or in another term, lately introduced, a ribose or a desoxyribose nucleic acid, a definite answer has not yet been offered. For the purpose of aquiring a clear knowledge of this problem many detailed in vestigations extending also to various bacilli are desired , notwith standing the fact that in this field of study many difficulties are naturally encountered, the foremost being to prepare a sufficient amount of investigation materials from bacteria, in whose cultiva tion enormous expense is involved, and secondly, to submit the crude product to a rigorous purification for detailed chemical analysis. The present paper reports the study of nucleic acid of typhoid bacillus of a mouse, which was conducted with the aim of obtaining material from bacilli in a high state of purity and to make a precise analysis of it in many respects, so that a com 355 356 S. -

THE TITRATABLE ACIDITY, Ph, AMMONIA and PHOSPHATES in the URINES of VERY YOUNG INFANTS BY
Arch Dis Child: first published as 10.1136/adc.22.112.200 on 1 December 1947. Downloaded from THE TITRATABLE ACIDITY, pH, AMMONIA AND PHOSPHATES IN THE URINES OF VERY YOUNG INFANTS BY R. A. McCANCE and M. A. VON FINCK (From the Medical Research Council, Department of Experimental Medicine, Cambridge, England; and Wuppertal, B.A.O.R.) It has now been established that for some time received. If there was a precipitate of urates in the after birth the kidney is still functionally immature. urines, it was customary to warm them before In many respects its actions differ from those of the removing the aliquots for the determinations of the titratable acidity, ammonia, phosphates, and total same organ in adult life. This is but one aspect of nitrogen, but the pH was usually determined before the physiology of infancy, but it is an important the urine was warmed. one in clinical medicine, and so illuminating have Specimens of urine were also obtained from Protected by copyright. the findings been that there is every reason to healthy British men and women and from healthy prosecute further studies of the organ in the early well nourished Germans. These urines were days of life. It has already been shown that the collected at various times throughout the day. glomerular filtration rate, the urea, and the sodium Some were early morning specimens. Aliquots were and chloride clearances are very low in newborn removed soon after the urines were passed for the babies, and that very young animals are unable to determination of pH, titratable acidity, and ammonia, and the remainder of the urine was concentrate their urines to the same extent as preserved under toluene if it was not possible to adults of the same species. -

Evaluation of Indicator and Antioxidant Activity of Hybrid Rose (Rosa Hybrida)
Satpal Bisht et al. / Journal of Pharmacy Research 2012,5(1),428-429 Research Article Available online through ISSN: 0974-6943 http://jprsolutions.info Evaluation of indicator and antioxidant activity of hybrid rose (Rosa hybrida) Satpal Bisht *, Rojita Mishra, Amrita Panda ,B.Praveen ,Koustava Panda and Tripti Sahu Department of Biotechnology,Roland Institute of Pharmaceutical Sciences,Berhampur- 760010, Orissa, India Received on:20-09-2011; Revised on: 15-10-2011; Accepted on:10-12-2011 ABSTRACT Rosa hybrida cv. Menu Pearl and cv. Cri Cri are hybrid tea roses. The petal extract of Rosa hybrida cv. Menu Pearl and cv. Cri Cri was tested as natural indicator in acid base titrations instead of the synthetic and other conventionally used indicators. Methanolic petal extract of cv. Menu Pearl when used as indicators showed coincidence point’s equivalent to phenolphthalein. Rose hips were analyzed by titration for the presence of vitamin C. Crude rose hips extract (unripe) of cv. Menu Pearl contained 325(w/w %) and cv. Cri Cri 112.5 (w/w%) Vitamin C. Even the flower petals contained Vitamin C twice the amount present in lemon and orange. Key Words: Rosa hybrida, Synthetic indicator, Vitamin C INTRODUCTION Rose is one of the most important ornamental flower immensely loved world slightly soft. Rose hips contain high concentration of ascorbic acid. Com- wide due to its fascinating beauty, mesmerizing fragrance, preventive and pletely ripe fruits contain ash, crude oil, crude energy, crude fiber, crude cosmetic medicinal benefits. The German chemist Richard Willstätter [1] protein, ascorbic acid, dimethyl sulfite and minerals including K, P, Mg, Ca made the first breakthrough towards the understanding of the chemical struc- and Fe [5]. -

Fingerprint Enhancement Revisited and the Effects Of
Chantal J. Frégeau,1 Ph.D.; Olivier Germain,2 B.S.; and Ron M. Fourney,1 Ph.D. Fingerprint Enhancement Revisited and the Effects of Blood Enhancement Chemicals on Subsequent Profiler Plus™ Fluorescent Short Tandem Repeat DNA Analysis of Fresh and Aged Bloody Fingerprints REFERENCE: Frégeau CJ, Germain O, Fourney RM. Fingerprint lected (i.e., Crowle’s Double Stain and Hungarian Red) slightly re- enhancement revisited and the effects of blood enhancement chem- duced the amplification efficiency of the longer STR loci in profiles icals on subsequent Profiler Plus™ fluorescent short tandem repeat of fresh and 7 to 14-day-old bloodprints. This suggests that long- DNA analysis of fresh and aged bloody fingerprints. J Forensic Sci term exposure to these chemicals possibly affects the integrity of the 2000;45(2):354–380. DNA molecules. This study indicates that significant evidence can be obtained from fresh or aged bloody fingerprints applied to a va- ABSTRACT: This study was aimed at determining the effect of riety of absorbent and nonabsorbent surfaces which are exposed to seven blood enhancement reagents on the subsequent Profiler different enhancement chemicals for short or long periods of time. Plus™ fluorescent STR DNA analysis of fresh or aged bloody fin- It also reaffirms that PCR STR DNA typing procedures are robust gerprints deposited on various porous and nonporous surfaces. and provide excellent results when used in concert with fluores- Amido Black, Crowle’s Double Stain, 1,8-diazafluoren-9-one cence-based detection assays after fingerprint identification has (DFO), Hungarian Red, leucomalachite green, luminol and ninhy- taken place.