The Action of the Phosphatases of Human Brain on Lipid Phosphate Esters by K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role and Significance of Sucrose-6-Phosphate Phosphatase in Regulating Sucrose Biosynthesis and Carbon Partitioning in Photosynthetic and Non-Photosynthetic Tissues
Role and significance of sucrose-6-phosphate phosphatase in regulating sucrose biosynthesis and carbon partitioning in photosynthetic and non-photosynthetic tissues Dissertation zur Erlangung des akademischen Grades Doktor der Naturwissenschaften -Dr. rer. nat.- vorgelegt der Mathematisch-Naturwissenschaftlich-Technischen Fakultät (mathematisch-naturwissenschaftlicher Bereich) der Martin-Luther-Universität Halle-Wittenberg von Herrn Shuai Chen geb. am 29. 08. 1975 in Shandong, Volksrepublik China 1. Gutachter: Prof. Dr. Uwe Sonnewald 2. Gutachter: Prof. Dr. Klaus Humbeck Halle (Saale), den 29. August 2005 urn:nbn:de:gbv:3-000008943 [http://nbn-resolving.de/urn/resolver.pl?urn=nbn%3Ade%3Agbv%3A3-000008943] Contents Contents 1 Introduction 1 1.1 Sink and source concept 1 1.2 Carbon partitioning between starch- and sucrose-synthesis in source leaves 2 1.3 Sucrose synthesis in source leaves 4 1.4 Phloem loading and long-distance transport of sucrose 6 1.5 Sucrose unloading and metabolism in sink organs 7 1.6 Sink regulation of photosynthesis and sugar signalling 10 1.7 Reversed genetics approaches for the identification of metabolic control steps 13 1.8 Chemical-inducible expression of transgenes to study plant metabolism 15 1.9 Scientific aims of this work 17 2 Materials and methods 18 2.1 Chemicals, enzymes and other consumables 18 2.2 Plant materials and growth conditions 18 2.2.1 Nicotiana tabacum 18 2.2.2 Solanum tuberosum 18 2.3 DNA cloning procedures 19 2.4 Oligonucleotides and DNA Sequencing 19 2.5 E. coli strains and plasmids 19 -

Phenolphthalein, 0.5% in 50% Ethanol Safety Data Sheet According to Federal Register / Vol
Phenolphthalein, 0.5% in 50% Ethanol Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Issue date: 12/17/2013 Revision date: 07/21/2020 Supersedes: 01/23/2018 Version: 1.5 SECTION 1: Identification 1.1. Identification Product form : Mixtures Product name : Phenolphthalein, 0.5% in 50% Ethanol Product code : LC18200 1.2. Recommended use and restrictions on use Use of the substance/mixture : For laboratory and manufacturing use only. Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem, Inc. 1010 Jackson's Pointe Ct. Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or +1-703-741-5970 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS US classification Flammable liquids Category 3 H226 Flammable liquid and vapor Carcinogenicity Category 1A H350 May cause cancer Reproductive toxicity Category 2 H361 Suspected of damaging the unborn child. (oral) Specific target organ toxicity (single exposure) Category 1 H370 Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS US labeling Hazard pictograms (GHS US) : Signal word (GHS US) : Danger Hazard statements (GHS US) : H226 - Flammable liquid and vapor H350 - May cause cancer H361 - Suspected of damaging the unborn child. (oral) H370 - Causes damage to organs (central nervous system, optic nerve) (oral, Dermal) Precautionary statements (GHS US) : P201 - Obtain special instructions before use. -

Student Safety Sheets Dyes, Stains & Indicators
Student safety sheets 70 Dyes, stains & indicators Substance Hazard Comment Solid dyes, stains & indicators including: DANGER: May include one or more of the following Acridine orange, Congo Red (Direct dye 28), Crystal violet statements: fatal/toxic if swallowed/in contact (methyl violet, Gentian Violet, Gram’s stain), Ethidium TOXIC HEALTH with skin/ if inhaled; causes severe skin burns & bromide, Malachite green (solvent green 1), Methyl eye damage/ serious eye damage; may cause orange, Nigrosin, Phenolphthalein, Rosaniline, Safranin allergy or asthma symptoms or breathing CORR. IRRIT. difficulties if inhaled; may cause genetic defects/ cancer/damage fertility or the unborn child; causes damages to organs/through prolonged or ENVIRONMENT repeated exposure. Solid dyes, stains & indicators including Alizarin (1,2- WARNING: May include one or more of the dihydroxyanthraquinone), Alizarin Red S, Aluminon (tri- following statements: harmful if swallowed/in ammonium aurine tricarboxylate), Aniline Blue (cotton / contact with skin/if inhaled; causes skin/serious spirit blue), Brilliant yellow, Cresol Red, DCPIP (2,6-dichl- eye irritation; may cause allergic skin reaction; orophenolindophenol, phenolindo-2,6-dichlorophenol, HEALTH suspected of causing genetic PIDCP), Direct Red 23, Disperse Yellow 7, Dithizone (di- defects/cancer/damaging fertility or the unborn phenylthiocarbazone), Eosin (Eosin Y), Eriochrome Black T child; may cause damage to organs/respiratory (Solochrome black), Fluorescein (& disodium salt), Haem- HARMFUL irritation/drowsiness or dizziness/damage to atoxylin, HHSNNA (Patton & Reeder’s indicator), Indigo, organs through prolonged or repeated exposure. Magenta (basic Fuchsin), May-Grunwald stain, Methyl- ene blue, Methyl green, Orcein, Phenol Red, Procion ENVIRON. dyes, Pyronin, Resazurin, Sudan I/II/IV dyes, Sudan black (Solvent Black 3), Thymol blue, Xylene cyanol FF Solid dyes, stains & indicators including Some dyes may contain hazardous impurities and Acid blue 40, Blue dextran, Bromocresol green, many have not been well researched. -

Phenolphthalein Is Pink in Base Acid–Base Indicators SCIENTIFIC
Phenolphthalein Is Pink in Base Acid–Base Indicators SCIENTIFIC Introduction Phenolphthalein is a large organic molecule used as an acid–base indicator. Phenolphthalein turns a bright red color as its solution becomes basic. In a strongly basic solution, this red color fades to colorless. Concepts • Acid–base indicators • Chemical equilibrium Background Phenolphthalein has the colorless structure shown in Figure 1 when the solution pH <8. As the solution becomes basic and the pH increases (pH 8–10), the phenolphthalein molecule (abbreviated H2P) loses two hydrogen ions to form the red- violet dianion (abbreviated P2–) shown in Figure 2. At a high pH, the P2– ions reacts with hydroxide ions to form the colorless POH3– ion. HO – – O –O O O OH O O– C C C – – + OH C O CO– 2 CO2 OH – CO2 O P2– Red 2– POH3– Colorless3– Figure 1. H2P is colorless. Figure 2. P is red. Figure 3. POH is colorless. 2– The colorless-to-red transition of H2P to P (Equation 1) is very rapid and the red color develops instantly when the pH reaches its transition range (pH 8–10). If the concentration of hydroxide ions remains high, the red P2– dianion will slowly combine with hydroxide ions to form a third species, POH3– (Equation 2), which is again colorless. The rate of this second reaction is much slower than the first and depends on the concentration of phenolphthalein and hydroxide ions. Thus, the color of the red P2– species will gradually fade in a basic solution. fast 2– + H2P → P + 2H Equation 1 Colorless Red slow P2– + OH– → POH3– Equation 2 Red Colorless Materials (for each demonstration) Hydrochloric acid solution, HCl, 3 M, 10 mL Beral-type pipet, disposable Phenolphthalein solution, 0.5%, 3mL Test tubes, borosilicate, 16 5 100mm, 3 Sodium hydroxide pellets, NaOH, 2 Test tube rack Sodium hydroxide solution, NaOH, 3 M, 5 mL Wash bottle Safety Precautions Hydrochloric acid solution is toxic and corrosive to eyes and skin tissue. -

Exploring Metagenomic Enzymes: a Novel Esterase Useful for Short-Chain Ester Synthesis
catalysts Article Exploring Metagenomic Enzymes: A Novel Esterase Useful for Short-Chain Ester Synthesis 1,2, 1,2, 1 Thaís Carvalho Maester y , Mariana Rangel Pereira z, Aliandra M. Gibertoni Malaman , Janaina Pires Borges 3,Pâmela Aparecida Maldaner Pereira 1 and Eliana G. M. Lemos 1,* 1 Department of Technology, São Paulo State University (UNESP), Jaboticabal, SP 14884-900, Brazil; [email protected] (T.C.M.); [email protected] (M.R.P.); [email protected] (A.M.G.M.); [email protected] (P.A.M.P.) 2 Institute of Biomedical Sciences (ICB III), University of São Paulo (USP), São Paulo, SP 05508-900, Brazil 3 Institute of Biosciences, Languages and Exact Sciences, Department of Chemistry and Environmental Sciences, São Paulo State University (UNESP), São José do Rio Preto, SP 15054-000, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-16-3209-7409 Current address: Supera Innovation and Technology Park, Ecobiotech Company, Ribeirão Preto, y SP 14056-680, Brazil. Current address: Department of Biochemistry, University of Cambridge, Cambridge CB2 1TN, UK. z Received: 13 August 2020; Accepted: 24 August 2020; Published: 23 September 2020 Abstract: Enzyme-mediated esterification reactions can be a promising alternative to produce esters of commercial interest, replacing conventional chemical processes. The aim of this work was to verify the potential of an esterase for ester synthesis. For that, recombinant lipolytic enzyme EST5 was purified and presented higher activity at pH 7.5, 45 ◦C, with a Tm of 47 ◦C. Also, the enzyme remained at least 50% active at low temperatures and exhibited broad substrate specificity toward p-nitrophenol esters 1 1 with highest activity for p-nitrophenyl valerate with a Kcat/Km of 1533 s− mM− . -

Association of Variation in the Sugarcane Transcriptome with Sugar Content Prathima P
Thirugnanasambandam et al. BMC Genomics (2017) 18:909 DOI 10.1186/s12864-017-4302-5 RESEARCH ARTICLE Open Access Association of variation in the sugarcane transcriptome with sugar content Prathima P. Thirugnanasambandam1,2†, Nam V. Hoang1,3†, Agnelo Furtado1, Frederick C. Botha4 and Robert J. Henry1,5* Abstract Background: Sugarcane is a major crop of the tropics cultivated mainly for its high sucrose content. The crop is genetically less explored due to its complex polyploid genome. Sucrose synthesis and accumulation are complex processes influenced by physiological, biochemical and genetic factors, and the growth environment. The recent focus on the crop for fibre and biofuel has led to a renewed interest on understanding the molecular basis of sucrose and biomass traits. This transcriptome study aimed to identify genes that are associated with and differentially regulated during sucrose synthesis and accumulation in the mature stage of sugarcane. Patterns of gene expression in high and low sugar genotypes as well as mature and immature culm tissues were studied using RNA-Seq of culm transcriptomes. Results: In this study, 28 RNA-Seq libraries from 14 genotypes of sugarcane differing in their sucrose content were used for studying the transcriptional basis of sucrose accumulation. Differential gene expression studies were performed using SoGI (Saccharum officinarum Gene Index, 3.0), SAS (sugarcane assembled sequences) of sugarcane EST database (SUCEST) and SUGIT, a sugarcane Iso-Seq transcriptome database. In total, about 34,476 genes were found to be differentially expressed between high and low sugar genotypes with the SoGI database, 20,487 genes with the SAS database and 18,543 genes with the SUGIT database at FDR < 0.01, using the Baggerley’s test. -

Changes in Phosphatidylinositol Metabolism in Response to Hyperosmotic Stress in Daucus Carota 1. Cells Grown in Suspension Culture'
Plant Physiol. (1993) 103: 637-647 Changes in Phosphatidylinositol Metabolism in Response to Hyperosmotic Stress in Daucus carota 1. Cells Grown in Suspension Culture' Myeon H. Cho, Stephen B. Shears, and Wendy F. BOSS* Department of Botany, North Carolina State University, Raleigh, North Carolina 27695-761 2 (M.H.C., W.F.B.); and Laboratory of Cellular and Molecular Pharmacology, National lnstitute of Environmental Health Sciences, Research Triangle Park, North Carolina 27709 (S.B.S) in the Samanea saman pulvini is there evidence for stimulus- Carrot (Daucus carota L.) cells plasmolyzed within 30 s after mediated turnover of polyphosphorylated inositol phospho- adding sorbitol to increase the osmotic strength of the medium lipids and inositol phosphates within the rapid time scale from 0.2 to 0.4 or 0.6 osmolal. However, there was no significant seen in animal cells (Morse et al., 1987). Although results change in the polyphosphorylated inositol phospholipids or inositol from in vitro studies and from IP3 microinjection in vivo have phosphates or in inositol phospholipid metabolism within 30 s of shown that Ir3 can release Ca2+from internal stores such as imposing the hyperosmotic stress. Maximum changes in phospha- vacuoles and tonoplast vesicles (Drcibak and Ferguson, 1985; tidylinositol 4-monophosphate (PIP) metabolism were detected at 5 min, at which time the cells appeared to adjust to the change in Schumaker and Sze, 1987; Ranjeva et al., 1988; Gilroy et al., osmoticum. There was a 30% decrease in [3H]inositol-labeledPIP. 1990), the complete pathway from a stimulus to Ca2+release The specific activity of enzymes involved in the metabolism of the has yet to be demonstrated in higher plants. -

Some Ultrastructural and Enzymatic Effects of Water Stress in Cotton (Gossypium Hirsutum L.) Leaves (Acid Phosphatase/Acid Lipase/Alkaline Lipase)
Proc. Nat. Acad. Sci. USA Vol. 71, No. 8, pp. 3243-3247, August 1974 Some Ultrastructural and Enzymatic Effects of Water Stress in Cotton (Gossypium hirsutum L.) Leaves (acid phosphatase/acid lipase/alkaline lipase) JORGE VIEIRA DA SILVA*, AUBREY W. NAYLOR, AND PAUL J. KRAMER Department of Botany, Duke University, Durham, North Carolina 27706 Contributed by Paul J. Kramer, May 30, 1974 ABSTRACT Water stress induced by floating discs cut boxylation of glycine occurs after lipase treatment of mito- from cotton leaves (Gossypium hirsutum L. cultivar chondria Stoneville) on a polyethylene glycol solution (water poten- (23). tial, -10 bars) was associated with marked alteration of Results, thus far obtained by indirect means, support the ultrastructural organization of both chloroplasts and hypothesis that water stress in drought sensitive species leads mitochondria. Ultrastructural organization of chloro- to hydrolytic activity that degrades not only storage products plasts was sometimes almost completely destroyed; per- but the structural framework of organelles such as ribosomes, oxisomes seemed not to be affected; and chloroplast ribosomes disappeared. Also accompanying water stress chloroplasts, and mitochondria. Ultrastructural and micro- was a sharp increase in activity of acid phosphatase chemical evidence is reported here that such deterioration [orthoplhosphoric-monoester phosplhohydrolase (acid opti- occurs in cotton (Gossypium hirsutum L. cv. Stoneville) during mum), EC 3.1.3.2], and acid and alkaline lipase [glycerol water stress. ester hydrolase EC 3.1.1.3] within chloroplasts. Only acid lipase activity was detected inside mitochondria of stressed MATERIALS AND METHODS discs. These alterations in cell organization and enzy- mology may account for at least part of the previously Cotton plants (Gossypium hirsutum L. -

MATERIAL SAFETY DATA SHEET Phenolphthalein
MATERIAL SAFETY DATA SHEET Phenolphthalein Section 1 Chemical Product and Company Identification MSDS Name: Phenolphthalein Catalog 147710000, 147711000, 147715000, 417180000, 417180025, 417181000, 41718 Numbers: 5000 Synonyms: Company Identification: Acros Organics BVBA Janssen Pharmaceuticalaan 3a 2440 Geel, Belgium Company Identification: (USA) Acros Organics One Reagent Lane Fair Lawn, NJ 07410 For information in the US, call: 800ACROS01 For information in Europe, call: +32 14 57 52 11 Emergency Number, Europe: +32 14 57 52 99 Emergency Number US: 2017967100 CHEMTREC Phone Number, US: 8004249300 CHEMTREC Phone Number, Europe: 7035273887 Section 2 Composition, Information on Ingredients CAS# Chemical Name: % EINECS# 77098 Phenolphthalein 2010047 Hazard Symbols: XN Risk Phrases: 22 40 Section 3 Hazards Identification EMERGENCY OVERVIEW Harmful if swallowed. Limited evidence of a carcinogenic effect. Potential Health Effects Eye: May cause eye irritation. Skin: May cause skin irritation. May be harmful if absorbed through the skin. Ingestion: Harmful if swallowed. May cause irritation of the digestive tract. Ingestion may cause fever, blood pressure increase and other unspecified vascular effects. Inhalation: May cause respiratory tract irritation. May be harmful if inhaled. Chronic: Limited evidence of a carcinogenic effect. Section 4 First Aid Measures Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Ingestion: Get medical aid. Wash mouth out with water. -

The Usage of Coal-Layered Double Oxide for Removal of Toxic Dye From
Journal of Metals, Materials and Minerals, Vol. 30, No. 4, pp. 45-50, 2020 The usage of CoAl−layered double oxide for removal of toxic dye from aqueous solution Pornnapa TONGCHOO1, Sonchai INTACHAI1,*, Prakaidao PANKAM2, Chomponoot SUPPASO2, and Nithima KHAORAPAPONG2 1 Department of Chemistry, Faculty of Science, Thaksin University, Phatthalung, 93210, Thailand 2 Department of Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand *Corresponding author e-mail: [email protected] Abstract Received date: 5 July 2020 CoAl−layered double oxide derived from calcination of CoAl−layered double hydroxide at Revised date 400C for 120 min, delivered the change in microstructure with increasing surface area. Spinel Co3O4 28 October 2020 was the majority in the product as confirmed by X-ray diffraction, and Fourier transform infrared and Accepted date: UV-visible spectroscopies. The calcined product, CoAl−layered double oxide resulted in significant 31 October 2020 adsorption capacity on dye removal superior to that of CoAl−layered double hydroxide. The adsorbed amounts of the diverse dyes increased as follows: methylene blue phenolphthalein methyl orange Keywords: orange II. The adsorption affinity between adsorbent and dyes relied on electrostatic interaction Layered double oxide; and physical adsorption. Orange II; Methyl orange; Phenolphthalein; Methylene blue 1. Introduction [11-13]. The usage of LDO on the adsorptive removal of various dyes is worth investigation. Nowadays, the quality of natural water resources is numerously In this study, the adsorption of four organic dyes including degenerative. One of the possibilities may be arisen from contaminating methyl orange, orange II, methylene blue, and phenolphthalein on chemical effluents including textile dyes [1]. -

Human Erythrocyte Acetylcholinesterase
Pediat. Res. 7: 204-214 (1973) A Review: Human Erythrocyte Acetylcholinesterase FRITZ HERZ[I241 AND EUGENE KAPLAN Departments of Pediatrics, Sinai Hospital, and the Johns Hopkins University School of Medicine, Baltimore, Maryland, USA Introduction that this enzyme was an esterase, hence the term "choline esterase" was coined [100]. Further studies In recent years the erythrocyte membrane has received established that more than one type of cholinesterase considerable attention by many investigators. Numer- occurs in the animal body, differing in substrate ous reviews on the composition [21, 111], immunologic specificity and in other properties. Alles and Hawes [85, 116] and rheologic [65] properties, permeability [1] compared the cholinesterase of human erythro- [73], active transport [99], and molecular organiza- cytes with that of human serum and found that, tion [109, 113, 117], attest to this interest. Although although both enzymes hydrolyzed acetyl-a-methyl- many studies relating to membrane enzymes have ap- choline, only the erythrocyte cholinesterase could peared, systematic reviews of this area are limited. hydrolyze acetyl-yg-methylcholine and the two dia- More than a dozen enzymes have been recognized in stereomeric acetyl-«: /3-dimethylcholines. These dif- the membrane of the human erythrocyte, although ferences have been used to delineate the two main changes in activity associated with pathologic condi- types of cholinesterase: (1) acetylcholinesterase, or true, tions are found regularly only with acetylcholinesterase specific, E-type cholinesterase (acetylcholine acetyl- (EC. 3.1.1.7). Although the physiologic functions of hydrolase, EC. 3.1.1.7) and (2) cholinesterase or erythrocyte acetylcholinesterase remain obscure, the pseudo, nonspecific, s-type cholinesterase (acylcholine location of this enzyme at or near the cell surface gives acylhydrolase, EC. -
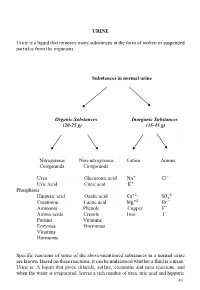
URINE Urine Is a Liquid That Removes Many Substances in the Form Of
URINE Urine is a liquid that removes many substances in the form of molten or suspended particles from the organism. Substances in normal urine Organic Substances Inorganic Substances (20-25 g) (35-45 g) Nitrogenous Non-nitrogenous Cation Anions Compounds Compounds Urea Glucuronic acid Na+ CI− Uric Acid Citric acid K+ Phosphates +2 −2 Hippuric acid Oxalic acid Ca SO4 Creatinine Lactic acid Mg+2 Br− Ammonia Phenols Copper F− Amino acids Cresols Iron I− Purines Vitamins Enzymes Hormones Vitamins Hormones Specific reactions of some of the above-mentioned substances in a normal urine are known. Based on these reactions, it can be understood whether a fluid is a urine. Urine is: A liquid that gives chloride, sulfate, creatinine and urea reactions, and when the water is evaporated, leaves a rich residue of urea, uric acid and hippuric 49 acid. Understanding whether a liquid is urine or not: •Determination of chloride, sulfate, urea, creatinine in liquid •Search for urea in the residual after the liquid has been evaporated Experiments will be carried out by sampling 2-3 ml into the tube. 1-Determination of chloride Liquid + a few drops of concentrated HNO3+ 2-3 drops of 5% AgNO3→ AgCl ↓ (white sediment) Nitric acid (HNO3 prevents the precipitation of phosphate and carbonate which could be precipitated by silver nitrate (AgNO3). 2-Determination of sulphate Liquid + several drops of 10% HCl + 2-3 drops BaCl2→ BaSO4↓ (white sediment) 3-Determination of urea Searched by two experiments: • Sodium hypobromite (NaOBr) • Using the enzyme urease a- Sodium