1.4. Northern Temperate and Boreal Forests in Ontario 11 1.4.1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plant List Bristow Prairie & High Divide Trail
*Non-native Bristow Prairie & High Divide Trail Plant List as of 7/12/2016 compiled by Tanya Harvey T24S.R3E.S33;T25S.R3E.S4 westerncascades.com FERNS & ALLIES Pseudotsuga menziesii Ribes lacustre Athyriaceae Tsuga heterophylla Ribes sanguineum Athyrium filix-femina Tsuga mertensiana Ribes viscosissimum Cystopteridaceae Taxaceae Rhamnaceae Cystopteris fragilis Taxus brevifolia Ceanothus velutinus Dennstaedtiaceae TREES & SHRUBS: DICOTS Rosaceae Pteridium aquilinum Adoxaceae Amelanchier alnifolia Dryopteridaceae Sambucus nigra ssp. caerulea Holodiscus discolor Polystichum imbricans (Sambucus mexicana, S. cerulea) Prunus emarginata (Polystichum munitum var. imbricans) Sambucus racemosa Rosa gymnocarpa Polystichum lonchitis Berberidaceae Rubus lasiococcus Polystichum munitum Berberis aquifolium (Mahonia aquifolium) Rubus leucodermis Equisetaceae Berberis nervosa Rubus nivalis Equisetum arvense (Mahonia nervosa) Rubus parviflorus Ophioglossaceae Betulaceae Botrychium simplex Rubus ursinus Alnus viridis ssp. sinuata Sceptridium multifidum (Alnus sinuata) Sorbus scopulina (Botrychium multifidum) Caprifoliaceae Spiraea douglasii Polypodiaceae Lonicera ciliosa Salicaceae Polypodium hesperium Lonicera conjugialis Populus tremuloides Pteridaceae Symphoricarpos albus Salix geyeriana Aspidotis densa Symphoricarpos mollis Salix scouleriana Cheilanthes gracillima (Symphoricarpos hesperius) Salix sitchensis Cryptogramma acrostichoides Celastraceae Salix sp. (Cryptogramma crispa) Paxistima myrsinites Sapindaceae Selaginellaceae (Pachystima myrsinites) -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Redmond Watershed Preserve King County, Washington
Vascular Plant List: Redmond Watershed Preserve King County, Washington Partial list covers plants found in the 800-acre watershed managed by the city of Redmond primarily as a nature preserve. Habitats include shady 90-year old forest, sunny disturbed utility corridors, ephemeral drainages, perennial streams, ponds and other wetlands. The preserve has 7 miles of trails (see external links below), most of which are multi-use and a couple of which are ADA accessible. List originally compiled by Fred Weinmann in February 2002. Ron Bockelman made additions in 2018. 128 species (83 native, 45 introduced) Directions: 21760 NE Novelty Hill Rd, Redmond, WA 98052 is the physical address. Drive 2.3 miles east on Novelty Hill Rd from its junction with Avondale Rd. Turn left at the entrance across from 218 Ave NE and continue to the parking lot, information kiosk, and restrooms. Ownership: City of Redmond Access: Open during daylight hours. No pets allowed. Permits: None External Links: https://www.wta.org/go-hiking/hikes/redmond-watershed-preserve https://www.alltrails.com/parks/us/washington/redmond-watershed-preserve Coordinates: 47.695943°, -122.051161° Elevation: 300 - 700 feet Key to symbols: * = Introduced species. ? = Uncertain identification. + = Species is represented by two or more subspecies or varieties in Washington; the species in this list has not been identified to subspecies or variety. ! = Species is not known to occur near this location based on specimen records in the PNW Herbaria database, and may be misidentified. # = Species name could not be resolved to an accepted name; the name may be misspelled. Numeric superscripts after a scientific name indicates the name was more broadly circumscribed in the past, and has since been split into two or more accepted taxa in Washington. -

Winter 2014-2015 (22:3) (PDF)
Contents NATIVE NOTES Page Fern workshop 1-2 Wavey-leaf basket Grass 3 Names Cacalia 4 Trip Report Sandstone Falls 5 Kate’s Mountain Clover* Trip Report Brush Creek Falls 6 Thank yous memorial 7 WEST VIRGINIA NATIVE PLANT SOCIETY NEWSLETTER News of WVNPS 8 VOLUME 22:3 WINTER 2014-15 Events, Dues Form 9 Judy Dumke-Editor: [email protected] Phone 740-894-6859 Magnoliales 10 e e e visit us at www.wvnps.org e e e . Fern Workshop University of Charleston Charleston WV January 17 2015, bad weather date January 24 2015 If you have thought about ferns, looked at them, puzzled over them or just want to know more about them join the WVNPS in Charleston for a workshop led by Mark Watson of the University of Charleston. The session will start at 10 A.M. with a scheduled end point by 12:30 P.M. A board meeting will follow. The sessions will be held in the Clay Tower Building (CTB) room 513, which is the botany lab. If you have any pressed specimens to share, or to ask about, be sure to bring them with as much information as you have on the location and habitat. Even photographs of ferns might be of interest for the session. If you have a hand lens that you favor bring it along as well. DIRECTIONS From the North: Travel I-77 South or 1-79 South into Charleston. Follow the signs to I-64 West. Take Oakwood Road Exit 58A and follow the signs to Route 61 South (MacCorkle Ave.). -

Autumn Willow in Rocky Mountain Region the Black Hills National
United States Department of Agriculture Conservation Assessment Forest Service for the Autumn Willow in Rocky Mountain Region the Black Hills National Black Hills National Forest, South Dakota and Forest Custer, South Dakota Wyoming April 2003 J.Hope Hornbeck, Carolyn Hull Sieg, and Deanna J. Reyher Species Assessment of Autumn willow in the Black Hills National Forest, South Dakota and Wyoming J. Hope Hornbeck, Carolyn Hull Sieg and Deanna J. Reyher J. Hope Hornbeck is a Botanist with the Black Hills National Forest in Custer, South Dakota. She completed a B.S. in Environmental Biology (botany emphasis) at The University of Montana and a M.S. in Plant Biology (plant community ecology emphasis) at the University of Minnesota-Twin Cities. Carolyn Hull Sieg is a Research Plant Ecologist with the Rocky Mountain Research Station in Flagstaff, Arizona. She completed a B.S. in Wildlife Biology and M.S. in Range Science from Colorado State University and a Ph.D. in Range and Wildlife Management (fire ecology) at Texas Tech University. Deanna J. Reyher is Ecologist/Soil Scientist with the Black Hills National Forest in Custer, South Dakota. She completed a B.S. degree in Agronomy (soil science and crop production emphasis) from the University of Nebraska – Lincoln. EXECUTIVE SUMMARY Autumn willow, Salix serissima (Bailey) Fern., is an obligate wetland shrub that occurs in fens and bogs in the northeastern United States and eastern Canada. Disjunct populations of autumn willow occur in the Black Hills of South Dakota. Only two populations occur on Black Hills National Forest lands: a large population at McIntosh Fen and a small population on Middle Boxelder Creek. -

(Lonicera Caerulea L.) by James K. Dawson a Th
Concentration and Content of Secondary Metabolites in Fruit and Leaves of Haskap (Lonicera caerulea L.) by James K. Dawson A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy In the Department of Plant Sciences ©James K. Dawson 2017 University of Saskatchewan All rights reserved. This thesis may not be reproduced in whole or in part, by photocopy or other means, without the permission of the author. PERMISSION TO USE In presenting this thesis/dissertation in partial fulfillment of the requirements for a Postgraduate degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I further agree that permission for copying of this thesis/dissertation in any manner, in whole or in part, for scholarly purposes may be granted by the professor or professors who supervised my thesis/dissertation work or, in their absence, by the Head of the Department or the Dean of the College in which my thesis work was done. It is understood that any copying or publication or use of this thesis/dissertation or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis/dissertation. Requests for permission to copy or to make other uses of materials in this thesis/dissertation in whole or part should be addressed to: Head of the Department of Plant Sciences Dean University of Saskatchewan College of Graduate Studies and Saskatoon, Saskatchewan S7N 5A8 OR University of Saskatchewan Canada 107 Administration Place Saskatoon, Saskatchewan S7N 5A2 Canada i ABSTRACT The University of Saskatchewan (UofS) has been conducting crosses of Lonicera caerulea and releasing genotypes for fruit production under the name “Haskap”. -
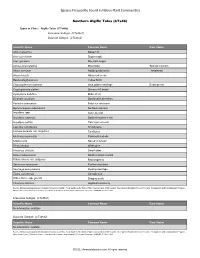
Algific Talus (Cts46)
Species Frequently Found in Native Plant Communities Southern Algific Talus (CTs46) Types in Class: Algific Talus (CTs46a) Limestone Subtype (CTs46a1) Dolomite Subtype (CTs46a2) Scientific Name Column1 Common Name Rare Status Abies balsamea Balsam fir Acer saccharum Sugar maple Acer spicatum Mountain maple Adoxa moschatellina Moschatel Special Concern Allium cernuum Nodding wild onion Threatened Arabis hirsuta Hairy rock cress Betula alleghaniensis Yellow birch Chrysosplenium iowense Iowa golden saxifrage Endangered Cryptogramma stelleri Slender cliff brake Cystopteris bulbifera Bulblet fern Dicentra cucullaria Dutchman's breeches Enemion biternatum False rue anemone Gymnocarpium robertianum Northern oak fern Impatiens spp. touch-me-not Impatiens capensis Spotted touch-me-not Impatiens pallida Pale touch-me-not Laportea canadensis Wood nettle Linnaea borealis var. longiflora Twinflower Mertensia paniculata Panicled bluebells Mitella nuda Naked miterwort Pinus strobus White pine Rhamnus alnifolia Dwarf alder Ribes hudsonianum Northern black currant Rubus idaeus var. strigosus Red raspberry Sambucus racemosa Red-berried elder Saxifraga pensylvanica Swamp saxifrage Taxus canadensis Canada yew Urtica dioica ssp. gracilis Stinging nettle Viburnum trilobum Highbush cranberry Source: Minnesota Department of Natural Resources (2005). Field Guide to the Native Plant Communities of Minnesota: The Eastern Broadleaf Forest Province. Ecological Land Classification Program, Minnesota County Biological Survey, and Natural Heritage and Nongame Research Program. MNDNR St. Paul, MN. Limestone Subtype (CTs46a1) Scientific Name Column1 Common Name Rare Status No information available Dolomite Subtype (CTs46a2) Scientific Name Column1 Common Name Rare Status No information available Source: Minnesota Department of Natural Resources (2005). Field Guide to the Native Plant Communities of Minnesota: The Eastern Broadleaf Forest Province. Ecological Land Classification Program, Minnesota County Biological Survey, and Natural Heritage and Nongame Research Program. -

Kenai National Wildlife Refuge Species List, Version 2018-07-24
Kenai National Wildlife Refuge Species List, version 2018-07-24 Kenai National Wildlife Refuge biology staff July 24, 2018 2 Cover image: map of 16,213 georeferenced occurrence records included in the checklist. Contents Contents 3 Introduction 5 Purpose............................................................ 5 About the list......................................................... 5 Acknowledgments....................................................... 5 Native species 7 Vertebrates .......................................................... 7 Invertebrates ......................................................... 55 Vascular Plants........................................................ 91 Bryophytes ..........................................................164 Other Plants .........................................................171 Chromista...........................................................171 Fungi .............................................................173 Protozoans ..........................................................186 Non-native species 187 Vertebrates ..........................................................187 Invertebrates .........................................................187 Vascular Plants........................................................190 Extirpated species 207 Vertebrates ..........................................................207 Vascular Plants........................................................207 Change log 211 References 213 Index 215 3 Introduction Purpose to avoid implying -

Conserving Plant Diversity in New England
CONSERVING PLANT DIVERSITY IN NEW ENGLAND A COLLABORATION OF CONSERVING PLANT DIVERSITY IN NEW ENGLAND A COLLABORATION OF AUTHORS Mark Anderson Director of Conservation Science, Eastern United States, The Nature Conservancy Michael Piantedosi Director of Conservation, Native Plant Trust William Brumback Director of Conservation Emeritus, Native Plant Trust MAP PRODUCTION Arlene Olivero WEB TOOL Melissa Clark DESIGN Rachel Wolff-Lander Kate Wollensak Freeborn The authors wish to thank the six state Natural Heritage programs for sharing their data and for their support. ©2021 Published June 2021 © Peter James CONTENTS EXECUTIVE SUMMARY ES-1 PART ONE: CONSERVING PLANT DIVERSITY 1-1 Background 1-2 • Plant Diversity and Resilience 1-2 • Global Strategy for Plant Conservation and Global Deal for Nature 1-7 • Secured Lands and GAP Status 1-9 • New England Flora and Rare Taxa 1-11 • Threats to Plant Diversity in New England 1-14 • Conservation Actions to Counter Threats to Plant Diversity 1-17 Conservation of Habitats and Important Plant Areas 1-21 Introduction 1-21 • Terminology 1-21 • Overview and Methods 1-22 Conservation of Habitats: Progress Toward Global and Regional Goals 1-26 • Matrix Forests 1-26 • Wetlands 1-30 • Patch-forming Habitats 1-33 • Risk of Conversion 1-36 Conservation of Important Plant Areas (IPAs) 1-37 • Definition and Location of IPAs 1-37 • Conservation Status and Progress Toward Goals 1-40 • Representation of Habitats in the IPAs 1-42 Conservation of Threatened Species 1-48 • Threatened Plants Conserved in situ 1-48 • Threatened Plants Conserved in ex situ Collections 1-49 Results and Recommendations 1-58 i CONTENTS continued PART TWO: STATUS REPORT AND MAPS 2-1 Overview 2-4 New England’s Terrestrial Habitats 2-7 Map Page Layout 2-13 Upland Habitats: Matrix Forest 2-16 Upland Habitats: Patch-forming Habitats 2-53 Wetland Habitats 2-80 PART THREE: SUPPORTING MATERIAL 3-1 Bibliography 3-2 Appendices 3-11 1. -

Total of 10 Pages Only May Be Xeroxed
CENTRE FOR NEWFOUNDLAND STUDIES TOTAL OF 10 PAGES ONLY MAY BE XEROXED (Without Author's Permission) ,, l • ...J ..... The Disjunct Bryophyte Element of the Gulf of St. Lawrence Region: Glacial and Postglacial Dispersal and Migrational Histories By @Rene J. Belland B.Sc., M.Sc. A thesis submitted to the School of Graduate Studies in partial fulfilment of the requirements for the degree of Doctor of Philosophy Department of Biology Memorial University of Newfoundland December, 1Q84 St. John's Newfoundland Abstract The Gulf St. Lawrence region has a bryophyte flora of 698 species. Of these 267 (38%) are disjunct to this region from western North America, eastern Asia, or Europe. The Gulf of St. Lawrence and eastern North American distributions of the disjuncts were analysed and their possible migrational and dispersal histories during and after the Last Glaciation (Wisconsin) examined. Based on eastern North American distribution patterns, the disjuncts fell into 22 sub elements supporting five migrational/ dispersal histories or combinations of these : (1) migration from the south, (2) migration from the north, (3) migration from the west, (4) survival in refugia, and (5) introduction by man. The largest groups of disjuncts had eastern North American distributions supporting either survival of bryophytes in Wisconsin ice-free areas of the Gulf of St. Lawrence or postglacial migration to the Gulf from the south. About 26% of the disjuncts have complex histories and their distributions support two histories. These may have migrated to the Gulf from the west and/or north, or from the west and/or survived glaciation in Gulf ice-free areas. -

A Review of Phytochemical Studies of Sorbus Species 213
INT. J. BOIL. BIOTECH., 16 (1): 211-220, 2019. GENUS SORBUS L.: A PHYTOCHEMICAL REVIEW Sadia Khan1*, Mehdi Hassan Kazmi1, Farah Inamullah1, Shagufta Afaq1, M. Zahid Farhad1, Sadia Ferheen2 and Sarwat Ismail3 1Department of Applied Chemistry, University of Karachi, Karachi-75270, Pakistan 2Pharmaceutical Research Centre, PCSIR Laboratories Complex Karachi, Pakistan 3PCSIR, Head office, 1-Constitution Avenue, G-5/2, Islamabad, Pakistan *corresponding author: [email protected] ABSTRACT The current review is focused on the phytochemical and pharmacological aspects of the genus Sorbus. Its numerous species have been utilized as a traditional medicine in the world. Despite, the therapeutic importance of genus Sorbus, out of 100 species only 13 species have been screened phytochemically. Up till now, around 150 secondary metabolites have been reported from this genus, which are summarized in this review. Keywords: Rosaceae, Sorbus, phytochemical, pharmacological potential. INTRODUCTION The Sorbus L. is a genus from the Rose family (Rosaceae). Sorbus comprises of 100 species among which many are medicinally important and are being used in the folklore medications all over the world (Bhattacharjee, 2003). Several taxa from Sorbus are found in customary and local medicines that are used as anti-diarrhea, diuretic, anti-inflammatory, anti-diabetic, vaso- protective, broncho- and vaso-relaxant, along with potent antioxidative qualities (Hukkanen et al., 2006, Olszewska and Michel, 2009, Olszewska et al., 2010, Olszewska, 2012; Perry and Metzger, 1980; Krishna, 1972; Jayaweera, 1982; Krachmal, 1980. The fruit of S. aucuparia L. is bitter in nature and having characteristics of, astringent, laxative, diuretic and cholagogue. Traditionally these are used as drugs in the form of tea, syrup, jelly or liqueur in Austria for the treatment of ailments related to respiratory tract, fever, infections, colds, flu, rheumatism and gout. -

(Lonicera L.) Genties Atstovų Genetinės Įvairovės Ir Filogenetiniai Tyrimai Dnr Ţymenų Metodais
VILNIAUS UNIVERSITETAS Donatas Naugţemys SAUSMEDŢIO (LONICERA L.) GENTIES ATSTOVŲ GENETINĖS ĮVAIROVĖS IR FILOGENETINIAI TYRIMAI DNR ŢYMENŲ METODAIS Daktaro disertacija Biomedicinos mokslai, biologija (01 B) Vilnius, 2011 Disertacija rengta 2006 – 2010 metais Vilniaus universitete. Mokslinis vadovas: prof. dr. Donatas Ţvingila (Vilniaus universitetas, biomedicinos mokslai, biologija – 01 B) Konsultantas: dr. Silva Ţilinskaitė (Vilniaus universitetas, biomedicinos mokslai, biologija – 01 B) 2 TURINYS SANTRUMPOS ..................................................................................................... 5 ĮVADAS ................................................................................................................. 7 I. LITERATŪROS APŢVALGA ......................................................................... 13 1. Sausmedţio genties apţvalga ....................................................................... 13 1.1. Lonicera L. genties sistematikos istorija ir problemos .......................... 15 1.2. Lonicera L. genties kilmė ...................................................................... 21 2. Molekuliniai ţymenys ir augalų filogenetiniai tyrimai ................................ 24 2.1. RAPD metodo taikymas augalų sistematikoje ...................................... 26 2.2. Chloroplastų DNR nekoduojančių specifinių regionų tyrimas sekoskaitos metodu .............................................................................................. 31 2.3. Lonicera L. genties filogenetikos molekuliniai tyrimai