Spatial Variability and Depuration of Tetrodotoxin in the Bivalve Paphies Australis from New Zealand T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

AEBR 114 Review of Factors Affecting the Abundance of Toheroa Paphies
Review of factors affecting the abundance of toheroa (Paphies ventricosa) New Zealand Aquatic Environment and Biodiversity Report No. 114 J.R. Williams, C. Sim-Smith, C. Paterson. ISSN 1179-6480 (online) ISBN 978-0-478-41468-4 (online) June 2013 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-resources/publications.aspx http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright - Ministry for Primary Industries TABLE OF CONTENTS EXECUTIVE SUMMARY ....................................................................................................... 1 1. INTRODUCTION ............................................................................................................ 2 2. METHODS ....................................................................................................................... 3 3. TIME SERIES OF ABUNDANCE .................................................................................. 3 3.1 Northland region beaches .......................................................................................... 3 3.2 Wellington region beaches ........................................................................................ 4 3.3 Southland region beaches ......................................................................................... -

Recent Trends in Marine Phycotoxins from Australian Coastal Waters
Review Recent Trends in Marine Phycotoxins from Australian Coastal Waters Penelope Ajani 1,*, D. Tim Harwood 2 and Shauna A. Murray 1 1 Climate Change Cluster (C3), University of Technology Sydney, Sydney, NSW 2007, Australia; [email protected] 2 Cawthron Institute, The Wood, Nelson 7010, New Zealand; [email protected] * Correspondence: [email protected]; Tel.: +61‐02‐9514‐7325 Academic Editor: Lucio G. Costa Received: 6 December 2016; Accepted: 29 January 2017; Published: 9 February 2017 Abstract: Phycotoxins, which are produced by harmful microalgae and bioaccumulate in the marine food web, are of growing concern for Australia. These harmful algae pose a threat to ecosystem and human health, as well as constraining the progress of aquaculture, one of the fastest growing food sectors in the world. With better monitoring, advanced analytical skills and an increase in microalgal expertise, many phycotoxins have been identified in Australian coastal waters in recent years. The most concerning of these toxins are ciguatoxin, paralytic shellfish toxins, okadaic acid and domoic acid, with palytoxin and karlotoxin increasing in significance. The potential for tetrodotoxin, maitotoxin and palytoxin to contaminate seafood is also of concern, warranting future investigation. The largest and most significant toxic bloom in Tasmania in 2012 resulted in an estimated total economic loss of ~AUD$23M, indicating that there is an imperative to improve toxin and organism detection methods, clarify the toxin profiles of species of phytoplankton and carry out both intra‐ and inter‐species toxicity comparisons. Future work also includes the application of rapid, real‐time molecular assays for the detection of harmful species and toxin genes. -

Physiological Effects and Biotransformation of Paralytic
PHYSIOLOGICAL EFFECTS AND BIOTRANSFORMATION OF PARALYTIC SHELLFISH TOXINS IN NEW ZEALAND MARINE BIVALVES ______________________________________________________________ A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy in Environmental Sciences in the University of Canterbury by Andrea M. Contreras 2010 Abstract Although there are no authenticated records of human illness due to PSP in New Zealand, nationwide phytoplankton and shellfish toxicity monitoring programmes have revealed that the incidence of PSP contamination and the occurrence of the toxic Alexandrium species are more common than previously realised (Mackenzie et al., 2004). A full understanding of the mechanism of uptake, accumulation and toxin dynamics of bivalves feeding on toxic algae is fundamental for improving future regulations in the shellfish toxicity monitoring program across the country. This thesis examines the effects of toxic dinoflagellates and PSP toxins on the physiology and behaviour of bivalve molluscs. This focus arose because these aspects have not been widely studied before in New Zealand. The basic hypothesis tested was that bivalve molluscs differ in their ability to metabolise PSP toxins produced by Alexandrium tamarense and are able to transform toxins and may have special mechanisms to avoid toxin uptake. To test this hypothesis, different physiological/behavioural experiments and quantification of PSP toxins in bivalves tissues were carried out on mussels ( Perna canaliculus ), clams ( Paphies donacina and Dosinia anus ), scallops ( Pecten novaezelandiae ) and oysters ( Ostrea chilensis ) from the South Island of New Zealand. Measurements of clearance rate were used to test the sensitivity of the bivalves to PSP toxins. Other studies that involved intoxication and detoxification periods were carried out on three species of bivalves ( P. -

Phylum MOLLUSCA Chitons, Bivalves, Sea Snails, Sea Slugs, Octopus, Squid, Tusk Shell
Phylum MOLLUSCA Chitons, bivalves, sea snails, sea slugs, octopus, squid, tusk shell Bruce Marshall, Steve O’Shea with additional input for squid from Neil Bagley, Peter McMillan, Reyn Naylor, Darren Stevens, Di Tracey Phylum Aplacophora In New Zealand, these are worm-like molluscs found in sandy mud. There is no shell. The tiny MOLLUSCA solenogasters have bristle-like spicules over Chitons, bivalves, sea snails, sea almost the whole body, a groove on the underside of the body, and no gills. The more worm-like slugs, octopus, squid, tusk shells caudofoveates have a groove and fewer spicules but have gills. There are 10 species, 8 undescribed. The mollusca is the second most speciose animal Bivalvia phylum in the sea after Arthropoda. The phylum Clams, mussels, oysters, scallops, etc. The shell is name is taken from the Latin (molluscus, soft), in two halves (valves) connected by a ligament and referring to the soft bodies of these creatures, but hinge and anterior and posterior adductor muscles. most species have some kind of protective shell Gills are well-developed and there is no radula. and hence are called shellfish. Some, like sea There are 680 species, 231 undescribed. slugs, have no shell at all. Most molluscs also have a strap-like ribbon of minute teeth — the Scaphopoda radula — inside the mouth, but this characteristic Tusk shells. The body and head are reduced but Molluscan feature is lacking in clams (bivalves) and there is a foot that is used for burrowing in soft some deep-sea finned octopuses. A significant part sediments. The shell is open at both ends, with of the body is muscular, like the adductor muscles the narrow tip just above the sediment surface for and foot of clams and scallops, the head-foot of respiration. -

Panopea Abrupta ) Ecology and Aquaculture Production
COMPREHENSIVE LITERATURE REVIEW AND SYNOPSIS OF ISSUES RELATING TO GEODUCK ( PANOPEA ABRUPTA ) ECOLOGY AND AQUACULTURE PRODUCTION Prepared for Washington State Department of Natural Resources by Kristine Feldman, Brent Vadopalas, David Armstrong, Carolyn Friedman, Ray Hilborn, Kerry Naish, Jose Orensanz, and Juan Valero (School of Aquatic and Fishery Sciences, University of Washington), Jennifer Ruesink (Department of Biology, University of Washington), Andrew Suhrbier, Aimee Christy, and Dan Cheney (Pacific Shellfish Institute), and Jonathan P. Davis (Baywater Inc.) February 6, 2004 TABLE OF CONTENTS LIST OF FIGURES ........................................................................................................... iv LIST OF TABLES...............................................................................................................v 1. EXECUTIVE SUMMARY ....................................................................................... 1 1.1 General life history ..................................................................................... 1 1.2 Predator-prey interactions........................................................................... 2 1.3 Community and ecosystem effects of geoducks......................................... 2 1.4 Spatial structure of geoduck populations.................................................... 3 1.5 Genetic-based differences at the population level ...................................... 3 1.6 Commercial geoduck hatchery practices ................................................... -

1 Oct 06 IPP Final
INTRODUCTION OF NEW STOCKS INTO THE QUOTA MANAGEMENT SYSTEM ON 1 OCTOBER 2006 CONSULTATION DOCUMENT 9 August 2005 TABLE OF CONTENTS INTRODUCTION........................................................................................................1 COCKLE, PIPI AND TUATUA IN FMA10................................................................15 DEEPWATER CLAM (PZL).....................................................................................17 KNOBBED WHELK (KWH).....................................................................................25 i ii INTRODUCTION 1 In accordance with sections 17B(3) and 19(7) of the Fisheries Act 1996 (the Act), the purpose of this document is to consult on behalf of the Minister of Fisheries on those species or stocks proposed for introduction into the Quota Management System (QMS) on 1 October 2006 (refer Table 1). The Ministry of Fisheries (MFish) requests that you provide your comments on the introduction of these species or stocks into the QMS, their proposed Quota Management Areas (QMAs), fishing year, unit of measure and assessment of the legislative criteria, as outlined in this document. 2 MFish requests that you provide your written comments in response to this consultation document no later than 16 September 2005. Your comments should be in response to the proposals for the species or stocks outlined in Table 1 in relation to: · The assessment of the legislative criteria; · The QMAs, including alternative options, for each stock; · The fishing year for each stock; and · The unit -

Conservation
3630 NEW ZEALAND GAZETTE No. 157 Fishery Quota Conservation Management Common Name Species Area Fisheries Act 1983 Atalacmea species, Scutus District Anglers (Auckland Acclimatisation species District) Notice 1987, Amendment No. 1 Mud snail Amphibola 2,7 & 8 crenata Pursuant to section 71 of the Fisheries Act 1983, the Auckland Mussels-blue Mytilus edulis New Zealand Acclimatisation Society amends the District Anglers (Auckland fisheries waters Acclimatisation District) Notice 1987, No. 164, Supplement to green-lipped Perna canaliculus New Zealand the New Zealand Gazette, 1987, pages 4508 and 4509 fisheries waters 1. Title and commencement-(1) This amending notice may horse Atrina zelandica New Zealand be cited as the District Anglers (Auckland Acclimatisation fisheries waters District) Notice 1987, Amendment No. 1, and shall be read Oyster-dredge Tiostrea Jutaria 1,2,7,8 & 9 together with and deemed part of the District Anglers rock Saccostrea 1&9 (Auckland Acclimatisation District) Notice 1987 (hereinafter glomerata referred to as the "principal notice"). Pipi Paphies australis New Zealand (2) This amending notice shall come into force on the 1st day fisheries waters of October 1988. Scallop- 2. Open season-The principal notice is amended by omitting New Zealand Pecten 1,3,4,5,6 & 9 from clause 3 (3) (i) the words, "except Parkinson's Lake". novaezelandiae Queen Chlamys 3,4,5 & 6 3. Closed season-Clause 4 of the principal notice is delicacula repealed and the following clause is substituted: Surf Clams including Dosinia New Zealand "4. Closed season-There shall be a closed season from the anus, Paphies fisheries waters 1st day of October 1988 to the 30th day of April 1989 (both donacina, Mactra days included) in respect of the taking of acclimatised fish discors, Mactra from the Awakino River upstream of the State Highway bridge murchisonii, at Mahoenui." Spisula 4. -

2017 SMALL BIVALVE FISHERY ASSESSMENT Venerupis Largillierti - Northern Zone, Georges Bay Katelysia Scalarina - Ansons Bay
2017 SMALL BIVALVE FISHERY ASSESSMENT Venerupis largillierti - Northern Zone, Georges Bay Katelysia scalarina - Ansons Bay John Keane and Caleb Gardner June 2017 Institute for Marine and Antarctic Studies, University of Tasmania, Private Bag 49, Hobart TAS 7001 Enquires should be directed to: Dr John Keane Institute for Marine and Antarctic Studies University of Tasmania Private Bag 49, Hobart, Tasmania 7001, Australia [email protected] Ph. (03) 6226 8265 Citation: Keane, J.P. and Gardner, C. (2017), 2017 Small Bivalve Fishery Assessment. Institute for Marine and Antarctic Studies Report. University of Tasmania, Hobart. 18 p. The authors do not warrant that the information in this document is free from errors or omissions. The authors do not accept any form of liability, be it contractual, tortious, or otherwise, for the contents of this document or for any consequences arising from its use or any reliance placed upon it. The information, opinions and advice contained in this document may not relate, or be relevant, to a reader’s particular circumstance. Opinions expressed by the authors are the individual opinions expressed by those persons and are not necessarily those of the Institute for Marine and Antarctic Studies (IMAS) or the University of Tasmania (UTas). The Institute for Marine and Antarctic Studies, University of Tasmania 2017. Copyright protects this publication. Except for purposes permitted by the Copyright Act, reproduction by whatever means is prohibited without the prior written permission of the Institute for Marine and Antarctic Studies. Small Bivalve Survey 2017 Executive Summary In 2017, stock assessments with total allowable commercial catch recommendations (TACC) ware conducted for the Georges Bay Northern Zone Venus Clam, Venerupis largillierti, fishery and the Ansons Bay Vongole, Katelysia scalarina, fishery. -

Support for Harvest Strategy Development in SA Lakes And
SUPPORT FOR HARVESTING STRATEGY DEVELOPMENT FOR SOUTH AUSTRALIA’S LAKES AND COORONG FISHERY FOR PIPI (DONAX DELTOIDES) Final report to the Fisheries Research and Development Corporation GJ Ferguson and TM Ward FRDC Project No. 2008/008 ISBN: 978-1-921563-56-0 April 2014 This report may be cited as: Ferguson, G.J., and Ward, T.M. (2014). Support for harvest strategy development in South Australia’s Lakes and Coorong Fishery for pipi (Donax deltoides). Final report to the Fisheries Research and Development Corporation. Prepared by the South Australian Research and Development Institute (Aquatic Sciences), Adelaide. FRDC Project No. 2008/008. 153pp. Date: 10 April 2014 Published by: South Australia Research and Development Institute © Copyright Fisheries Research and Development Corporation and South Australia Research and Development Institute, 2014 This work is copyright. Except as permitted under the Copyright Act 1968 (Cth), no part of this publication may be reproduced by any process, electronic or otherwise, without the specific written permission of the copyright owners. Information may not be stored electronically in any form whatsoever without such permission. Disclaimer The authors warrant that they have taken all reasonable care in producing this report. The report has been through the SARDI internal review process, and has been formally approved for release by the Research Chief, Aquatic Sciences. Although all reasonable efforts have been made to ensure quality, SARDI does not warrant that the information in this report is free from errors or omissions. SARDI does not accept any liability for the contents of this report or for any consequences arising from its use or any reliance placed upon it. -

Bivalvia: Hiatellidae), in Patagonia Variación En El Patrón Reproductivo a Escala Local De La Almeja Panopea, Panopea Abbreviata (Bivalvia: Hiatellidae), En Patagonia
Revista de Biología Marina y Oceanografía Vol. 51, Nº2: 359-371, agosto 2016 DOI 10.4067/S0718-19572016000200013 ARTICLE Local scale variation in the reproductive pattern of the southern geoduck, Panopea abbreviata (Bivalvia: Hiatellidae), in Patagonia Variación en el patrón reproductivo a escala local de la almeja panopea, Panopea abbreviata (Bivalvia: Hiatellidae), en Patagonia Paula C. Zaidman1,3, Marina A. Kroeck1, Silvina Van der Molen2,3, Gabriela Williams2,3, Leilen Gracia-Villalobos2,3, Erica Oehrens-Kissner1 and Enrique M. Morsan1 1Centro de Investigación Aplicada y Transferencia Tecnológica en Recursos Marinos Alte. Storni (CIMAS), Universidad Nacional del Comahue, Provincia de Río Negro, CONICET Guemes 1030, San Antonio Oeste, Rio Negro, Argentina 2Centro para el Estudio de Sistemas Marinos, CONICET, Boulevard Brown 2850 (U9120ACV), Puerto Madryn, Chubut, Argentina 3Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Av. Rivadavia 1917, (C1033AAJ) CABA, Argentina. *Corresponding author: [email protected] Resumen.- Durante el 2007 se realizaron estudios para explorar la variabilidad espacial a escala local, del ciclo reproductivo de Panopea abbreviata en 3 poblaciones presentes en los golfos Nord-patagónicos (El Sótano y Puerto Lobos, en el Golfo San Matías, y Punta Conos en el Golfo San José), Argentina. Estudios previos determinaron que P. abbreviata presenta un patrón reproductivo, en ambos sexos, caracterizado por una continua proliferación y evacuación de gametos durante todo el año. Sin embargo, el uso de indicadores cuantitativos (distribución de frecuencia de diámetros ovocitarios, ovocitos por campo ocular y área ovocitaria relativa) para las hembras permitió observar una ligera estacionalidad. Las variaciones en el ciclo reproductivo fueron relacionadas con el régimen anual de temperatura de cada sitio. -
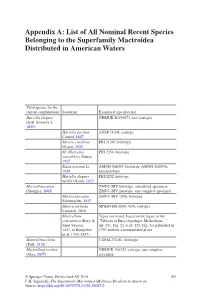
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype;