Antimicrobial Chemotherapy
A. History:
1. Antibiotics known for long time= chemicals produced by certain organisms that killed other organism. 2. Ehrlich's "magic bullet": 1909, discovered "Salvarsan", chemical used to treat syphillis. Ehrlich stressed Selective toxicity as key factor in success.
B. Structural analogues as drugs
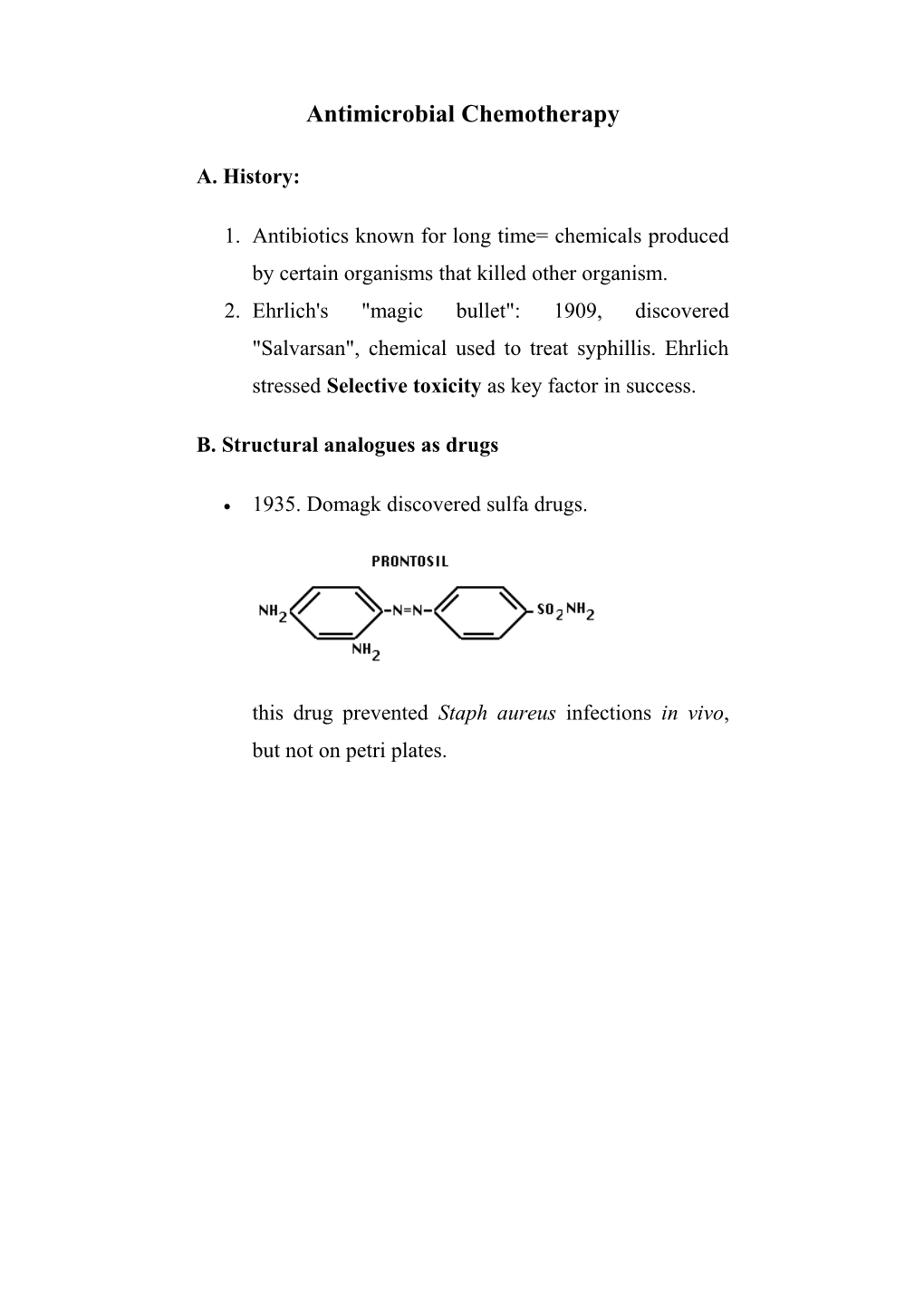
1935. Domagk discovered sulfa drugs.
this drug prevented Staph aureus infections in vivo, but not on petri plates. C. Antibiotics:
After Sulfa drug success, more search for drugs.
Penicillin was discovered in 1929, not commercially developed until WWII.
Since then major search for antibiotics. Found in 3 major groups of microorganisms: 1. Certain molds (Penicillium, Cephalosporium). e.g. penicillin,cephalosporin 2. Certain strains of Bacillus e.g. bacitracin 3. Many strains of Actinomycetes (soil bacteria that grow in long filamentous masses). Especially from Genus Streptomycetes. e.g. streptomycin. Majority of antibiotics come from these organisms.
Antibiotics may have:
1. a cidal (killing) effect or 2. a static (inhibitory) effect on a range of microbes.
The range of bacteria or other microorganisms that is affected by a certain antibiotic is expressed as its spectrum of action.
Antibiotics effective against procaryotes which kill or inhibit a wide range of Gram-Negative and Gram-Positive bacteria are said to be broad spectrum.
If effective mainly against Gram-Negative or Gram-Positive bacteria, they are narrow spectrum.
If effective against a single organism or disease, they are referred to as limited spectrum. A clinically-useful antibiotic should have as many of these characteristics as possible.
1. It should have a wide spectrum of activity with the ability to destroy or inhibit many different species of pathogenic organisms. 2. It should be nontoxic to the host and without undesirable side effects. 3. It should be nonallergenic to the host. 4. It should not eliminate the normal flora of the host. 5. It should be able to reach the part of the human body where the infection is occurring. 6. It should be inexpensive and easy to produce. 7. It should be chemically-stable (have a long shelf-life).
Types of Antimicrobial Agents and their Primary Modes of Action
1. Cell Wall antibiotics
Penicillins. First widely available drug, introduced in 1945. Contains ß-lactam ring. Benzylpenicillin (Penicillin G) was first natural isolate Activity: binds to enzymes that carry out transpeptidation linkage in bacterial cells. Unique target in procaryotes.
Note beta-lactam ring -- this is critical for activity.
INITIAL PRODUCTION: 1-10 ug/ ml. Gradual strain improvement over years, today 85,000 ug/ml!!!
BenzylPenicillin G -- low activity vs Gram-Negative, ß-lactamase sensitive
Modifications: side chain can be chemically modified. e.g. 1. Methicillin, Oxacillin -- acid stable, ß- lactamase resistant Methicillin
Oxacillin
2. Ampicillin -- broader spectrum (esp. vs Gram- Negative), acid stable, ß-lactamase sensitive. 3. Carbenicillin -- broader spectrum (esp. vs Pseudomonads), acid stable but not effective orally, ß-lactamase sensitive
Other antibiotics active against growth of cell wall: Cephalosporins, Cycloserine, Bacitracin. 1. Cephalosporins (e.g. cefoxitin, cephalothin) are ß-lactam antibiotics, dihydrothiazine ring instead of thiazolidine ring. Broader spectrum than penicillins, low toxicity. Relatively resistant to pencillinase. 2. Bacitracin does not block transpeptidation, but previous step in wall synthesis. Limited to topical application, because of severe toxic reactions.
2. Inhibitors of protein synthesis
Aminoglycosides: (have amino sugars linked by glycosidic bonds). streptomycin, gentamicin, kanamycin, tobramycin, amikacin. Used mainly for Gram-Negative infections. Not very effective against anaerobes or Gram-Positive. Bind to 30s ribosomal unit, block protein synthesis. Also cause misreading of mRNA. Useful for a number of diseases. Different degrees of toxicity (e.g. Gentamicin is very toxic, only used for severe infections) . Amikacin is best antibiotic for Gram-Negative rod hospital infections, because such infections (nosocomial) are often caused by strains with R- factors, resistant to many common antibiotics. Generally group is used as reserve antibiotics, when others fail.
Tetracyclines. (4 ring system) Also bind to 30S subunit,block protein synthesis. Effective against variety of pathogenic bacteria- broad spectrum. Together with ß-lactam antibiotics, the most important group commercially. Glycopeptide antibiotics. E.g. Vancomycin.
o Two antibiotics commercially available: vancomycin & teicoplanin.
o Vancomycin (Vancocin) -- worldwide use. Discovered 1956, produced by fungus Amycolatopsis orientalis, from Indonesia. Drug has relatively high toxicity and requires IV administration. Currently the drug of last resort for treating methicillin-resistant staph infections.
o Teicoplanin (Targocid) -- another glycopeptide, has longer half-life than vancomycin, can be administered only once a day by intramuscular or IV. Is effective against some vancomycin- resistant strains.
Macrolide antibiotics. E.g. Erythromycin. Large lactone rings connected to sugar groups. Binds to 50S ribosome, blocks protein synthesis. Most active vs. Gram-Positive organisms, eg. Strep. pyogenes. Now routinely applied to eyes of newborns to prevent gonnorhaea and chlamydia from infecting eye. 3. Effects on nucleic acid
affect the synthesis of DNA or RNA, or can bind to DNA or RNA so that their messages cannot be read can block the growth of cells
Two nucleic acid synthesis inhibitors which have selective activity against procaryotes and some medical utility are the quinolones and rifamycins.
Nalidixic acid is a synthetic chemotherapeutic agent which has activity mainly against Gram-negative bacteria. Nalidixic acid belongs to a group of compounds called quinolones. Nalidixic acid is a bactericidal agent. the main use of nalidixic acid is in treatment of lower urinary tract infections (UTI).
Fluoroquinolone (ciprofloxacin): have a broadened spectrum against Gram-positive bacteria and was recently touted as the drug of choice for treatment and prophylaxis of anthrax, which is caused by a Gram-positive bacillus. Rifampicin is a semisynthetic derivative of rifamycin that is active against Gram-positive bacteria (including Mycobacterium tuberculosis) and some Gram-negative bacteria. The table below is a summary of the classes of antibiotics and their properties including their biological source and mode of action.
Table 1. Classes of antibiotics and their properties
Spectrum Biological Chemical class Examples (effective Mode of action source against) Inhibits steps in Penicillium Beta-lactams cell wall Penicillin G, notatum and Gram-positive (penicillins and (peptidoglycan) Cephalothin Cephalosporium bacteria cephalosporins) synthesis and species murein assembly Inhibits steps in Gram-positive cell wall Semisynthetic Ampicillin, and Gram- (peptidoglycan) penicillin Amoxycillin negative synthesis and bacteria murein assembly Gram-positive Clavamox is "Suicide" Streptomyces and Gram- Clavulanic Acid clavulanic acid inhibitor of beta- clavuligerus negative plus amoxycillin lactamases bacteria Inhibits steps in Gram-positive cell wall Chromobacter and Gram- Monobactams Aztreonam (peptidoglycan) violaceum negative synthesis and bacteria murein assembly Inhibits steps in Gram-positive cell wall Streptomyces and Gram- Carboxypenems Imipenem (peptidoglycan) cattleya negative synthesis and bacteria murein assembly Gram-positive Inhibit translation Streptomyces and Gram- Aminoglycosides Streptomycin (protein griseus negative synthesis) bacteria Gram-positive and Gram- Inhibit translation Micromonospora Gentamicin negative (protein species bacteria esp. synthesis) Pseudomonas Glycopeptides Vancomycin Streptomyces Gram-positive Inhibits steps in orientales bacteria, esp. murein Staphylococcus (peptidoglycan) aureus biosynthesis and assembly
Gram-positive and Gram- Inhibits Streptomyces negative translation Lincomycins Clindamycin lincolnensis bacteria esp. (protein anaerobic synthesis) Bacteroides Gram-positive bacteria, Gram- negative Inhibits Streptomyces bacteria not translation Macrolides Erythromycin erythreus enterics, (protein Neisseria, synthesis) Legionella, Mycoplasma Damages Bacillus Gram-negative Polypeptides Polymyxin cytoplasmic polymyxa bacteria membranes Inhibits steps in murein Gram-positive Bacitracin Bacillus subtilis (peptidoglycan) bacteria biosynthesis and assembly Inactivate Streptomyces membranes Polyenes Amphotericin Fungi nodosus containing sterols Inactivate Streptomyces membranes Nystatin Fungi (Candida) noursei containing sterols Gram-positive and Gram- Inhibits Streptomyces negative transcription Rifamycins Rifampicin mediterranei bacteria, (bacterial RNA Mycobacterium polymerase) tuberculosis Tetracyclines Tetracycline Streptomyces Gram-positive Inhibit translation species and Gram- (protein negative synthesis) bacteria, Rickettsias Gram-positive and Gram- negative Inhibit translation Semisynthetic Doxycycline bacteria, (protein tetracycline Rickettsias synthesis) Ehrlichia, Borrelia Gram-positive Inhibits Streptomyces and Gram- translation Chloramphenicol Chloramphenicol venezuelae negative (protein bacteria synthesis)
D. Assessment of bacterial sensitivity
Anti-microbial activity in-vitro Disc diffusion tests 1. add test bacteria to small amount of melted agar 2. pour over surface of nutrient agar plate, let gel 3. add paper disks with known dose of antibiotic to surface 4. incubate: antibiotic will diffuse into medium as cells grow 5. examine plate: look for clear zones around disk where growth is inhibited 6. measure diameter of clear zones: consult table to find if this is clinically useful
Minimum inhibitory concentration (MIC) assay:
It give a more detailed assessment of bacterial susceptibility. Suspension of the test bacteria are inoculated overnight with doubling dilutions of antibiotics; the lowest concentration that inhibits growth is MIC.
Epsilomenter or ‘E’ test:
New techniques for direct determination of MIC 1. A gradual increasing concentration of antibiotic is fixed along plastic strip which applied to the surface of inoculated agar plate. 2. after overnight incubation tear drop shaped inhibition zone is seen . 3. the zone edge intersects the graded test strip at the MIC of the antibiotics
Example of organisms and antimicrobial agents for which susceptibility is usually predictable:
Organism Anti-microbial agents normally active Streptococci Penicillin, erythromycin, vancomycin Enterococci Ampicillin , vancomycin Anaerobic cocci Penicillin, erythromycin Staphylococci Oxacillin, clindamycin, gentamicin, vancomycin Haemophilus influenzae Amoxiclav, tetracycline, erythromycin E. coli & Proteus Gentamicin, ciprofloxacin, co-amoxclav mirabilis Pseudomonas Gentamicin, ciprofloxacin, imipenem, aeruginosa Bacteroides fragilis Cefoxitin, co-amoxiclav Rickettsiae & Tetracycline, macrolides, rifampicin Chlamydia mycoplasmas Tetracycline, erythromycin Candida albicans Nystatin, amphotericin B, azoles Herpes simplex virus Aciclovir
Examples of organisms and anti-microbial agents for which resistance is usually predicted.
Organism Antimicrobial agent not normally active Streptococci & Aminoglycosides, nalidixic acid, Enterococci aztreonam Staphylococci Most penicillin, nalidixic acid, aztreonam E. Coli Penicillin, vancomycin, erythromycin Klebsiella spp Most penicillin, erythromycin Pseudomonas Most penicillin and cephalosporins, aeruginosa erythromycin Anaerobes Aminoglycosides, azetreonam, nalidixic acid
Drug Resistance:
Drug resistance first noted in Japanese hospitals; serious increase in bacterial strains resistant to variety of standard antibiotics.
Since then, many examples of drug resistance developing. Ex: gonorrhea initially treated by pencillin. But pencillin-resistant strains now account for more than 25% of isolates, must use different antibiotic. Note: antibiotic resistance has always been present; frozen bacterial cultures from before WW II have been shown to include drug resistant individuals even though antibiotics weren't yet used by humans. Conclude that antibiotics are natural part of biological activity, not surprising that some resistance should have developed in course of evolution.
What is new, and different, is rate of development of resistance. Some disesase, like TB, never easy to treat even with the few antibiotics that were effective. Now drug resistant strains appearing, TB becoming much harder to treat.
Different ways for bacteria to develop drug resistance
1. mutations affecting cell surface can affect entry of drug
o prevents entry of drug into cell 2. receptor normally used by drug altered- no binding.
o example: mutations can affect drug target in cell (e.g. slight change in ribosomal RNA can change affinity of ribosomes for erythromycin) 3. bacteria or plasmids can produce enzymes which inactivate drug; e.g. pencillinases hydrolyze ß-lactam ring.
o Plasmids = small, circular DNA elements that reside in bacterial cells, duplicate separately from bacterial chromosome.
o Some plasmids carry genes for antibiotic resistance (enzymes that degrade antibiotic). Called R-plasmids. Have been found for most classes of antibiotics.
o When antibiotics are in use, most bacteria are killed. If R-plasmid exists, can be transferred to other cells, resistance spreads through population. Result: new population is resistant to drug.
o Note: possible for a single plasmid to carry multiple drug resistance genes, spread all of these as a single unit! 4. plasmid encoded drug pump
o production of protein "pumps" to pump drug out of cell
Ways to deal with antibiotic resistance
1. higher dose, different antibiotic, more than one drug simultaneously 2. also restraint by physicians and control (no over the counter use) 3. CORRECT use of drug. Most people take drugs improperly, miss doses, allow conditions that favor selection of drug resistant mutants.
Other anti-microbial agent:-
Antifungal Drugs
1. Polyenes, such as nystatin and amphotericin B, combine with plasma membrane sterols and are fungicidal. 2. Azoles interfere with sterol synthesis and are used to treat cutaneous and systemic mycoses.
3. Griseofulvin interferes with eukaryotic cell division and is used primarily to treat skin infections caused by fungi.
Antiviral Drugs
1. Amantadine blocks penetration or uncoating of influenza A virus.
2. Nucleoside and nucleotide analogs such as acyclovir, AZT, ddI, and ddC inhibit DNA or RNA synthesis.
3. Protease inhibitors, such as indinavir and saquinavir, block activity of an HIV enzyme essential for assembly of a new viral coat.