Date: Name: CHEM Activity: Average Atomic Mass Candium What’s the Average Atomic Mass?
Purpose Overall. The overall purpose of this activity is to learn how to determine the average atomic mass of an element. The average atomic mass is defined as the weighted average mass for all of the isotopes of that element. The ‘weighted’ takes into account the abundance of each element.
Specific. Just as most elements have several isotopes, the “element” candium comes in tw0 isotopic forms. In this activity, you will determine the average atomic mass for the element Candium.
Background Review – Atomic Structure The number of protons (p+) determines the identity of the atom. The number of electrons(e–) determines the charge of the atom. The number of neutrons (no) determines the isotope of the atom. And, mass number is the number of protons + the number of neutrons. (Electrons have a mass of ~1/2000 and are not included in the mass number.)
An example is chlorine-37, with a charge of –1. It has 17p+, 18e– & 20no, and has a mass 35 number of 37. Its symbol is 17 Cl .
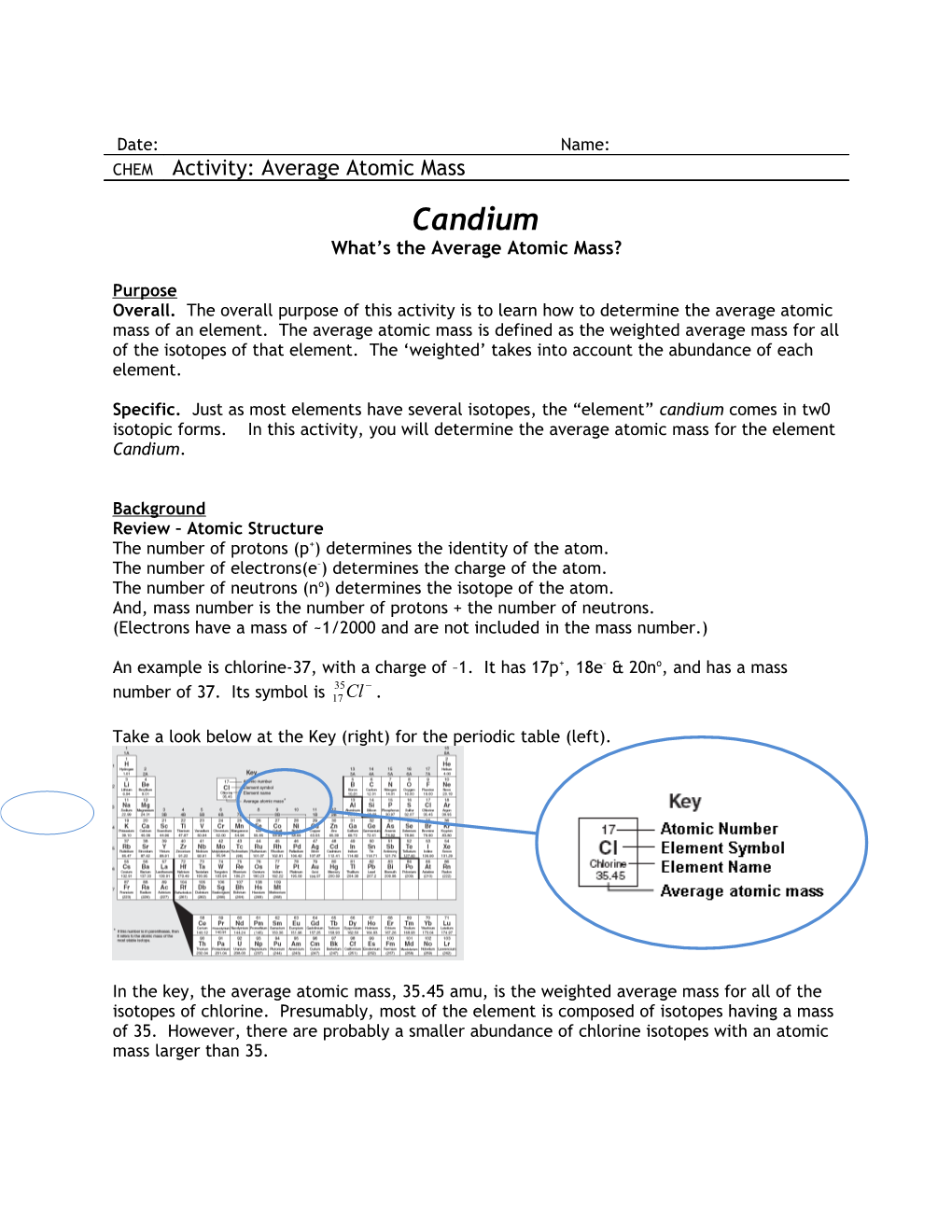
Take a look below at the Key (right) for the periodic table (left).
In the key, the average atomic mass, 35.45 amu, is the weighted average mass for all of the isotopes of chlorine. Presumably, most of the element is composed of isotopes having a mass of 35. However, there are probably a smaller abundance of chlorine isotopes with an atomic mass larger than 35. Chemistry Candium: Average Atomic Mass p. 2
In this lab, the two isotopes of Candium are:
Isotope-1: Isotope-2: Chemistry Candium: Average Atomic Mass p. 3
Procedure 1. Separate your element into two isotopes.
2. Find the total mass of each isotope group and record your calculations in the table below.
DATA:
[A] [B] [C = B/A] Isotope Number of Mass (grams) Average Isotope Mass “Atoms” Candium isotope 1
Candium isotope 2
TOTAL NUMBER OF CANDIUM ATOMS:
3. Complete the following table.
[C] [D = (A/total) * 100%] [E = D/100%] [F = C * E] Average mass Percent Relative Contribution of of each isotope abundance of abundance of mass of each each isotope each isotope isotope to the (candy piece) (candy type) (candy type) weighted average Candium isotope 1
Candium isotope 2
TOTAL: n/a
AVERAGE ATOMIC MASS (sum of contribution for each isotope) is in the above space
4. Find the average mass of all isotopes. ______
5. Class average for relative mass. ______
6. Calculate the percent error*. ______
Observed Accepted %error x100 Accepted
* Your calculated value is the OBSERVED, the class averages would be the ACCEPTED Chemistry Candium: Average Atomic Mass p. 4
CHALLENGE You will be given a sealed container that holds a mixture of ten M&M’s and Skittles. Your container might hold any particular combination of the two “isotopes.” Your task is to determine the isotopic composition of the element “Candium” without opening the container.
To illustrate how this is done, we can consider a mixture of our two isotopes of “Candium.” If your container 9 M&M’s and one skittle, it would have a mass of 9 M&M’s PLUS Skittle. We would mathematically represent the TOTAL MASS of your container to be:
TOTAL MASS = (# of M&M’s x mass of M&M) + (# of Skittles x mass of one Skittle)
Since we know our sample has ten “Atoms” we can say that:
= # of M&M’s and (10 – ) = # of Skittles
Combine these two equations to solve in algebraic terms:
TOTAL MASS = (c x mass of one M&M) + ((10 – ) x mass of one Skittle)
Unknown Sample
Mass of Container w/ Candium
Mass of Container
Mass of Candium (SUBTRACT)
# M&M’s in Sample
# Skittles in Sample
% Composition of Candium: M&M’s (# M&M’s * Average Mass of M&M’s/Total Mass of Sample) * 100%
%Composition of Candium: Skittles
(# Skittles * Average Mass of Skittles/Total Mass of Sample) * 100%
Show Calculations Below:
Analysis Chemistry Candium: Average Atomic Mass p. 5
1. Define isotope.
2. Explain the difference between percent abundance and relative abundance.
3. How does the average atomic mass differ from the relative atomic mass?
5. Determine the average atomic masses of the following elements. Show all your organized work below.
Magnesium (symbol ____)
Mass Number Exact Mass Percent Abundance 24 23.985042 78.99
25 24.985837 10.00
26 25.982593 11.01 Chemistry Candium: Average Atomic Mass p. 6
Molybdenum (symbol ___ )
Mass Number Exact Mass Percent Abundance 92 91.906808 14.84
94 93.905085 9.25
95 94.905840 15.92
96 95.904678 16.68
97 96.906020 9.55
98 97.905406 24.13
100 99.907477 9.63