Synergistic Passivation of Fly Ash and TMT on Heavy Metals in Sewage Sludge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Legislation for the Reuse of Biosolids on Agricultural Land in Europe: Overview
sustainability Review Legislation for the Reuse of Biosolids on Agricultural Land in Europe: Overview Maria Cristina Collivignarelli 1 , Alessandro Abbà 2, Andrea Frattarola 1, Marco Carnevale Miino 1 , Sergio Padovani 3, Ioannis Katsoyiannis 4,* and Vincenzo Torretta 5 1 Department of Civil and Architectural Engineering, University of Pavia, via Ferrata 1, 27100 Pavia, Italy; [email protected] (M.C.C.); [email protected] (A.F.); [email protected] (M.C.M.) 2 Department of Civil, Environmental, Architectural Engineering and Mathematics, University of Brescia, via Branze 43, 25123 Brescia, Italy; [email protected] 3 ARPA Lombardia, Pavia Department, via Nino Bixio 13, 27100 Pavia, Italy; [email protected] 4 Department of Chemistry, Laboratory of Chemical and Environmental Technology, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece 5 Department of Theoretical and Applied Sciences, University of Insubria, via G.B. Vico 46, 21100 Varese, Italy; [email protected] * Correspondence: [email protected] Received: 17 September 2019; Accepted: 25 October 2019; Published: 29 October 2019 Abstract: The issues concerning the management of sewage sludge produced in wastewater treatment plants are becoming more important in Europe due to: (i) the modification of sludge quality (biological and chemical sludge are often mixed with negative impacts on sludge management, especially for land application); (ii) the evolution of legislation (landfill disposal is banned in many European countries); and (iii) the technologies for energy and material recovery from sludge not being fully applied in all European Member States. Furthermore, Directive 2018/851/EC introduced the waste hierarchy that involved a new strategy with the prevention in waste production and the minimization of landfill disposal. -

Energy Recovery from Sewage Sludge: the Case Study of Croatia
energies Article Energy Recovery from Sewage Sludge: The Case Study of Croatia Dinko Đurđevi´c 1,* , Paolo Blecich 2 and Željko Juri´c 1 1 Energy Institute Hrvoje Požar, 10000 Zagreb, Croatia; [email protected] 2 Faculty of Engineering, University of Rijeka, 51000 Rijeka, Croatia; [email protected] * Correspondence: [email protected] Received: 26 April 2019; Accepted: 16 May 2019; Published: 20 May 2019 Abstract: Croatia produced 21,366 tonnes of dry matter (DM) sewage sludge (SS) in 2016, a quantity expected to surpass 100,000 tonnes DM by 2024. Annual production rates for future wastewater treatment plants (WWTP) in Croatia are estimated at 5.8–7.3 Nm3/people equivalent (PE) for biogas and 20–25 kgDM/PE of sewage sludge. Biogas can be converted into 12–16 kWhel/PE of electricity and 19–24 kWhth/PE of heat, which is sufficient for 30–40% of electrical and 80–100% of thermal autonomy. The WWTP autonomy can be increased using energy recovery from sewage sludge incineration by 60% for electricity and 100% of thermal energy (10–13 kWhel/PE and 30–38 kWhth/PE). However, energy for sewage sludge drying exceeds energy recovery, unless solar drying is performed. 2 The annual solar drying potential is estimated between 450–750 kgDM/m of solar drying surface. The lower heating value of dried sewage sludge is 2–3 kWh/kgDM and this energy can be used for assisting sludge drying or for energy generation and supply to WWTPs. Sewage sludge can be considered a renewable energy source and its incineration generates substantially lower greenhouse gases emissions than energy generation from fossil fuels. -

Global Atlas of Excreta, Wastewater Sludge, and Biosolids Management: Moving Forward the Sustainable and Welcome Uses of a Global Resource
GLOBAL ATLAS OF EXCRETA, WASTEWATER SLUDGE, AND BIOSOLIDS MANAGEMENT: MOVING FORWARD THE SUSTAINABLE AND WELCOME USES OF A GLOBAL RESOURCE GLOBAL ATLAS OF EXCRETA, WASTEWATER SLUDGE, AND BIOSOLIDS MANAGEMENT: MOVING FORWARD THE SUSTAINABLE AND WELCOME USES OF A GLOBAL RESOURCE For further information please contact: Graham P. Alabaster Chief, Section I, Water, Sanitation and Infrastructure Branch Human Settlements Financing Division UN-HABITAT P.O. Box 30030, Nairobi 00100, Kenya Tel: +254 20 762 3054 Fax: +254 20 762 3588 [email protected] www.unhabitat.org Ronald J. LeBlanc Chairman – Greater Moncton Sewerage Commission Président – Commission d’épuration des eaux usées du Grand Moncton 355 chemin Hillsborough Road Riverview, New Brunswick (Nouveau-Brunswick) CANADA E1B 1S5 Tel: +1 506-387-7977 Fax: +1 506-387-7389 [email protected] www.gmsc.nb.ca Edited by: Ronald J. LeBlanc, Peter Matthews, Roland P. Richard Graphic design and layout: Daniel Vilnersson Cover pho to © Metrogro/Nikki Stefonick World map on chapter title pages is based on UN map no. 4136 rev. 5, September 2006. © United Nations Printed by HS/1030/08E ISBN: 978-92-1-132009-1 DISCLAIMER The designations employed and the presentation of the material in this atlas do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations, the Greater Moncton Sewerage Com- mission and the Editors concerning the legal status of any country, territory, city or area, or of its authorities, or concerning delimitation of its frontiers or boundaries, or regarding its economic system or degree of development. The analysis and conclusions of this report do not necessarily reflect the views of the United Nations or its Member States, the Greater Moncton Sewerage Commission and the Editors. -

Sludge Treatment and Disposal Biological Wastewater Treatment Series
Sludge Treatment and Disposal Biological Wastewater Treatment Series The Biological Wastewater Treatment series is based on the book Biological Wastewater Treatment in Warm Climate Regions and on a highly acclaimed set of best selling textbooks. This international version is comprised by six textbooks giving a state-of-the-art presentation of the science and technology of biological wastewater treatment. Titles in the Biological Wastewater Treatment series are: Volume 1: Wastewater Characteristics, Treatment and Disposal Volume 2: Basic Principles of Wastewater Treatment Volume 3: Waste Stabilisation Ponds Volume 4: Anaerobic Reactors Volume 5: Activated Sludge and Aerobic Biofilm Reactors Volume 6: Sludge Treatment and Disposal Biological Wastewater Treatment Series VOLUME SIX Sludge Treatment and Disposal Cleverson Vitorio Andreoli, Marcos von Sperling and Fernando Fernandes (Editors) Published by IWA Publishing, Alliance House, 12 Caxton Street, London SW1H 0QS, UK Telephone: +44 (0) 20 7654 5500; Fax: +44 (0) 20 7654 5555; Email: [email protected] Website: www.iwapublishing.com First published 2007 C 2007 IWA Publishing Copy-edited and typeset by Aptara Inc., New Delhi, India Printed by Lightning Source Apart from any fair dealing for the purposes of research or private study, or criticism or review, as permitted under the UK Copyright, Designs and Patents Act (1998), no part of this publication may be reproduced, stored or transmitted in any form or by any means, without the prior permission in writing of the publisher, or, in the case of photographic reproduction, in accordance with the terms of licences issued by the Copyright Licensing Agency in the UK, or in accordance with the terms of licenses issued by the appropriate reproduction rights organization outside the UK. -

SEWAGE SLUDGE DISPOSAL in the Federal Republic of Germany
SEWAGE SLUDGE DISPOSAL in the Federal Republic of Germany German Environment Agency Imprint Publisher: German Environment Agency Sections III 2.4 Waste Technology, Waste Technology Transfer and III 2.5 Monitoring Methods, Waste Water Management Post Box 14 06 D-06813 Dessau-Roßlau Phone: +49 340-2103-0 [email protected] Internet: www.umweltbundesamt.de /umweltbundesamt.de /umweltbundesamt.de/umweltbundesamt /umweltbundesamt /umweltbundesamt /umweltbundesamt Authors: Authors:Andrea Roskosch, Patric Heidecke Andrea Roskosch, Patric Heidecke Contributors: Contributors:Claus-Gerhard Bannick, Simone Brandt, Maja Bernicke, Claus-GerhardClaudia Dienemann, Bannick, Marcus Simone Gast, Brandt, Maximilian Maja Bernicke,Hofmeier, ClaudiaChristian Dienemann, Kabbe, Kathrin Marcus Schwirn, Gast, InesMaximilian Vogel, DorisHofmeier, Völker, ChristianBenjamin Kabbe,Wiechmann Kathrin Schwirn, Ines Vogel, Doris Völker, Benjamin Wiechmann Design: Design:Atelier Hauer + Dörfler GmbH, Berlin Atelier Hauer + Dörfler GmbH, Berlin Publication as a pdf: Publicationwww.umweltbundesamt.de/publikationen as a pdf: www.umweltbundesamt.de/publikationen Photo credits: PhotoTitel: shutterstock.com credits: Titel:stock.adobe.com: shutterstock.com Markus Bormann, cpt212, Gordon Grand, stock.adobe.com:stu12; shutterstock.com Markus Bormann, cpt212, Gordon Grand, stu12; shutterstock.com As of May 2018, Asfully of revised May 2018, edition fully revised edition ISSN (print) 2363-8311 ISSN (print)(online) 2363-8311 2363-832X] ISSN (online) 2363-832X] SEWAGE SLUDGE DISPOSAL -

Management, Use, and Disposal of Sewage Sludge - S
WASTE MANAGEMENT AND MINIMIZATION – Management, Use, and Disposal of Sewage Sludge - S. R. Smith MANAGEMENT, USE, AND DISPOSAL OF SEWAGE SLUDGE S. R. Smith Department of Civil and Environmental Engineering, Imperial College, London, UK Keywords: Agriculture, biosolids, incineration, landfill, nutrients, organic contaminants, pathogens, potentially toxic elements, recycling, sewage sludge. Contents 1. Introduction 2. Best Practicable Environmental Option 3. Hazard Assessment Critical Control Point and Sludge Management Audits 4. Sludge Production 5. Sludge Treatment 6 Routes for the Disposal or Recycling of Sewage Sludge 7. Environmental Aspects of Beneficial Re-use 8. Conclusions and Future Development Acknowledgements Glossary Bibliography Biographical Sketch Summary Sewage sludge is a by-product of urban wastewater treatment. Sludge production in Europe and the USA has increased dramatically with the implementation of environmental programs to improve the quality of discharges from wastewater treatment plant. Environmental legislation has also banned the disposal of sludge by sea dispersal requiring all the sludge from sewage treatment to be disposed of on land. The amount of sludge generated in the EU is expected to increase by 50% to about 10 million t ds y-1 by 2005 compared to 1992 and approximately 7 million t ds y-1 are currently produced in the USA. Germany, UK, France, and Italy contribute the largest amounts of sludge equivalent to 70% of total EU production and Spain is also forecast to become a major sludge producer in future. MesophilicUNESCO anaerobic digestion is the most– popularEOLSS sludge stabilization process and 50% of sludge produced in the EU is treated by this method. Thermophilic aerobic digestion is alsoSAMPLE widely employed and about CHAPTERS 20% of sludge in the EU is stabilized aerobically. -

Lesson B5 SEWAGE SLUDGE TREATMENT
EMWATER E-LEARNING COURSE PROJECT FUNDED BY THE EUROPEAN UNION LESSON B5: SEWAGE SLUDGE TREATMENT Lesson B5 SEWAGE SLUDGE TREATMENT Authors: Holger Pabsch Claudia Wendland Institute of Wastewater Management Hamburg University of Technology Keywords sewage sludge, stabilisation, sludge thickening, sludge dewatering, sludge pumping, digestion Page 1 of 23 EMWATER E-LEARNING COURSE PROJECT FUNDED BY THE EUROPEAN UNION LESSON B5: SEWAGE SLUDGE TREATMENT Table of Content Overview and summary of this Lesson 3 1. Sewage sludge quantity and characteristics 4 2. Sludge pumping systems 5 3. Sludge stabilisation 7 3.1 Simultaneous aerobic stabilisation 7 3.2 Mesophilic anaerobic digestion 8 3.3 Aerobic digestion 11 3.4 Alkaline Stabilisation 12 4. Thickening/ dewatering of Sludge 14 4.1 Dimensioning of a static thickener 15 4.2 Dewatering 16 5. Sludge Disposal and Agricultural Utilisation 20 6. Exercises 21 7. References and further Information 23 Page 2 of 23 EMWATER E-LEARNING COURSE PROJECT FUNDED BY THE EUROPEAN UNION LESSON B5: SEWAGE SLUDGE TREATMENT Overview and summary of this Lesson Sludge originates from the process of treatment of wastewater and is separated from the treatment process by sedimentation or flotation. Sewage sludge consists of water and solids that can be divided into mineral and organic solids. The quantity and characteristics of sludge depend very much on the treatment processes. Most of the pollutants that enter the wastewater get adsorbed to the sewage sludge. Therefore, sewage sludge contains pathogens (and heavy metals, many organic pollutants pesticides, hydrocarbons etc. if the sewage contains industrial influence). Sludge is, however, rich in nutrients such as nitrogen and phosphorous and contains valuable organic matter that is useful if soils are depleted or subject to erosion. -

Redalyc.Sewage Sludge Compost Fertilizer Effect on Maize Yield And
Revista Internacional de Contaminación Ambiental ISSN: 0188-4999 [email protected] Universidad Nacional Autónoma de México México Delgado Arroyo, Ma. del Mar; Porcel Cots, Miguel Ángel; Miralles de Imperial Hornedo, Rosario; Beltrán Rodríguez, Eulalia Ma.; Beringola Beringola, Luisa; Martín Sánchez, José Valero Sewage sludge compost fertilizer effect on maize yield and soil heavy metal concentration Revista Internacional de Contaminación Ambiental, vol. 18, núm. 3, 2002, p. 0 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=37018305 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Rev. Int. Contam. Ambient. 18 (3) 147-150, 2002 SEWAGE SLUDGE COMPOST FERTILIZER EFFECT ON MAIZE YIELD AND SOIL HEAVY METAL CONCENTRATION Ma. del Mar DELGADO ARROYO, Miguel Ángel PORCEL COTS, Rosario MIRALLES DE IMPERIAL HORNEDO, Eulalia Ma. BELTRÁN RODRÍGUEZ, Luisa BERINGOLA BERINGOLA and José Valero MARTÍN SÁNCHEZ Departamento de Medio Ambiente, INIA. Apdo. 8111. 28080 Madrid, Spain, e-mail: [email protected] (Recibido abril de 2001, aceptado julio de 2002) Key words: sewage sludge, reusing, fertilization, production, heavy metals, maize ABSTRACT Studies were conducted to determine the use of an organic residue (sewage sludge compost) for four years (1996-1999), to study effects of sewage compost on crop yield and chemical proper- ties of soil under field condition. Productivity studies showed that the greatest growth is ob- tained in mixed II treatment (12000 kg/ha sewage sludge compost plus 350 kg/ha urea) with 20 % more than mineral fertilization, followed by mixed I (8000 kg/ha sewage sludge compost plus 350 kg/ha urea) with 10 % more than mineral fertilization. -

Irradiated Sewage Sludge for Application to Cropland
IAEA-TECDOC-1317 Irradiated sewage sludge for application to cropland Results of a co-ordinated research project organized by the Joint FAO-IAEA Division of Nuclear Techniques in Food and Agriculture October 2002 The originating Section of this publication in the IAEA was: Soil and Water Management & Crop Nutrition Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria IRRADIATED SEWAGE SLUDGE FOR APPLICATION TO CROPLAND IAEA, VIENNA, 2002 IAEA-TECDOC-1317 ISBN 92–0–117102–1 ISSN 1011–4289 © IAEA, 2002 Printed by the IAEA in Austria October 2002 FOREWORD Modern urban societies produce large volumes of sewage, which are transported through a network of underground sewers to wastewater treatment plants, where one or more stages of physical, biological and chemical treatment are imposed. Considerable tonnages of aerobically, and sometimes anaerobically, digested sludge are produced, and treated or untreated effluent is discharged to lagoons, waterways or the ocean. The disposal of sewage sludge is a major issue for municipal authorities. There are increasing legislative restrictions in many countries on disposal methods (e.g. incineration, landfill, composting) including surface application to agricultural land. Sludge can either be viewed as a dangerous waste requiring expensive disposal procedures, or it can be seen as a resource for possible use in agriculture as a soil conditioning agent and a source of plant nutrients. Untreated sewage sludge presents a public-health hazard as it contains human pathogens, including bacteria, viruses and other harmful organisms. Although it has been demonstrated that an appropriate dose of gamma-irradiation can eliminate human parasites and bacterial pathogens from sewage sludge, there is still public concern about the presence of viruses, as well as heavy metals and toxic organic compounds from industrial sources that could enter the food chain if sludge is applied to croplands. -

Land Application of Sewage Sludge and Domestic Septage EPA/625/R-95/001 September 1995
United States Office of Research and EPA/625/R-95/001 Environmental Protection Development September 1995 Agency Washington, DC 20460 1EPA Process Design Manual Land Application of Sewage Sludge and Domestic Septage EPA/625/R-95/001 September 1995 Process Design Manual Land Application of Sewage Sludge and Domestic Septage U.S. Environmental Protection Agency Office of Research and Development National Risk Management Research Laboratory Center for Environmental Research Information Cincinnati, Ohio Notice This document has been reviewed in accordance with the U.S. Environmental Protection Agency’s peer and administrative review policies and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. ii Contents Page Chapter 1 Introduction 1.1 Overview . 1 1.2 Sewage Sludge Regulations. 1 1.3 Objectives of Manual . 2 1.4 Scope of Manual. 3 Chapter 2 Overview of Sewage Sludge Land Application Practices 2.1 Introduction . 5 2.2 Application to Agricultural Lands . 5 2.2.1 Purpose and Definition . 5 2.2.2 Advantages of Agricultural Land Application . 5 2.2.3 Limitations of Agricultural Land Application. 7 2.3 Application to Forest Lands . 7 2.3.1 Purpose and Definition . 7 2.3.2 Advantages of Forest Land Application. 7 2.3.3 Limitations of Forest Land Application . 8 2.4 Land Application at Reclamation Sites. 8 2.4.1 Purpose and Definition . 8 2.4.2 Advantages of Land Application at Reclamation Sites . 9 2.4.3 Limitations of Land Application at Reclamation Sites. 9 2.5 Land Application at Public Contact Sites, Lawns, and Home Gardens . -
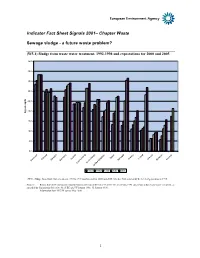
Sewage — a Future Waste Problem?
Indicator Fact Sheet Signals 2001– Chapter Waste Sewage sludge - a future waste problem? (W5.1) Sludge from waste water treatment, 1992-1998 and expectations for 2000 and 2005 45,0 40,0 35,0 30,0 25,0 20,0 Kg perKg capita 15,0 10,0 5,0 0,0 l in e tria ds c ay land uga land us ran in Spa ort Ire F Sweden A F Greece Norw Denmark Germany P Belgium Luxembourg Netherlan United Kingdom 1992 1995 1998 2000 2005 (W5.1) Sludge from waste water treatment 1992 to 1998 and forecast for 2000 and 2005. Member States sorted by the level of generation in 1995. Sources: Report from the Commission: Implementation of Council Directive 91/271/EEC of 21 May 1991 concerning urban waste water treatment, as amended by Commission Directive 98/15/EC of 27 February 1998. 15 January 1999. Information from ETC/W survey May 2000 1 (W5.2) Treatment of sewage sludge, selected EEA member countries, 1998 45,0 40,0 35,0 30,0 25,0 Kg per20,0 capita 15,0 10,0 5,0 0,0 Denmark Germany : Ã7UHDWPHQWÃRIÃVHZDJHÃVOXGJHÃVHOHFWHGÃ(($ÃPHPEHUÃFRXQWULHVÃÃLuxembourg à 6RXUFHÃ(7&:ÃVXUYH\Ã0D\ÃÃDQGÃLQIRUPDWLRQÃIURPà November 1998, following directive 86/278/EEC on sewage sludgeFinland à (W5.3) Contamination of sewage sludge, selected EEA member countries 1995 to 1997 Portugal Mercury Austria Total landfilling 2,5 Netherlands 2,0 1,5 1,0 Norway 0,5 Total recycling Mg 0,0per kg Phosfor United Kingdom report from Member States to the Commission concerning sewage sludge treatment, Germany Total incineration Spain Denmark France France Ireland Finland Belgium Greece Greece 1995 Ireland 1996 Luxembourg 1997 Norway Sweden UK 2 Cadmium 3,0 2,5 2,0 1,5 1,0 0,5 0,0 Mg per kg phosfor kg per Mg y e n c d d n n n UK la la ourg den rma ra n re b e F Fi Greece I Norway we G Denmark S Luxem 1995 1996 1997 Lead 200 150 100 50 0 Mg per kg phosfor nd UK la way reland r France Fin Greece I Germany Denmark embourg No Sweden ux L 1995 1996 1997 NOTE: VERTICAL AXIS TITLES – milligrams per kg dry matter Note: Average content reported by member countries. -
Biosolids Management Handbook Epa Region Viii
BIOSOLIDS MANAGEMENT HANDBOOK EPA REGION VIII 999 18th St., Denver, CO 80202 Mr. Robert Brobst PART 1 C BIOSOLIDSBIOSOLIDS REGULATIONSREGULATIONS Federal regulations newly published in the Federal Register can be difficult to understand. Therefore, an "easy-to-read" summary of 40 CFR Part 503 has been developed by EPA and has been included in this handbook for your use (Section 1.1). See also Part 503 Implementation Guidance (EPA 833-R-95-001), 1995. All of the information and requirements found in 40 CFR Part 503 are included in the provided summary. However, special attention should be paid to the Land Application and Surface Disposal sections and to the corresponding Pathogen and Vector Attraction Reduction requirements, since these practices are the most commonly used in EPA Region 8. Section 1.2 is a paper that was presented at an international conference in 1994. It provides the recent history of biosolids management, improvements in biosolids quality, and improvements made in equipment, processes and management practices. With this background in place, the author discusses the implementation of the 503 regulations, the problems associated with the aging infrastructure in the U.S., and some predictions for future biosolids management trends. Section Topic Page 1.1 Summary of 40 CFR Part 503 Standards for the Use or Disposal of Sewage Sludge .........................................1.1-1 1.2 United States Regulations and Practical Experience on Biosolids Reuse and Disposal.................. 1.2-1 1- 1 BIOSOLIDS MANAGEMENT HANDBOOK SECTION 1.1 C SUMMARYSUMMARY OFOF 4040 CFRCFR PARTPART 503503 STANDARDSSTANDARDS FORFOR THETHE USEUSE OROR DISPOSAL OF SEWAGE SLUDGE Robert K.