Chemistry 103b Name______Spring 2008- Exam 1 /15 Dr. Pollard You Must Show All Work For Credit /25 CIRCLE YOUR ANSWERS /25
0 order- [A]t = -kt + [A]o /15 st 1 order- ln[A]t = -kt + ln[A]o nd /10 2 order- 1/[A]t = kt + 1/[A]o ln k2/k1 = (Ea/R)(1/T2 - 1/T1) /10 R = 8.314J/mol K /100
1) True or False: (2) The half-life of a 2nd order process does not depend on the initial amount.
2) True or False: (2) A reaction with a large equilibrium constant is always fast.
3) (3) Consider the following reaction
8A(g) + 5B(g) --> 8C(g) + 6D(g) If [C] is increasing at the rate of 4.0 mol L-1s-1, at what rate is [D] changing?
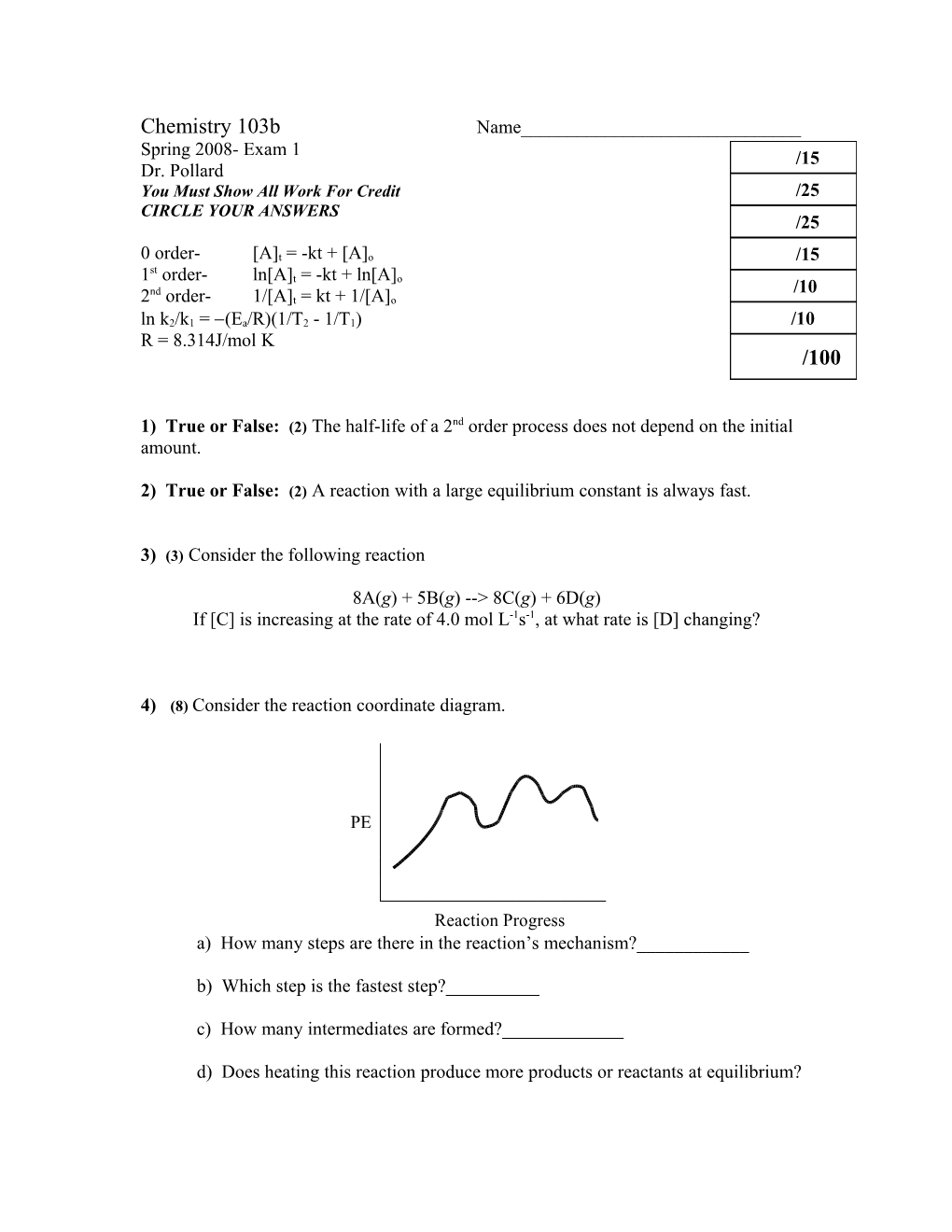
4) (8) Consider the reaction coordinate diagram.
PE
Reaction Progress a) How many steps are there in the reaction’s mechanism?______
b) Which step is the fastest step?______
c) How many intermediates are formed?______
d) Does heating this reaction produce more products or reactants at equilibrium? 5) (25 total) Consider the following reaction and rate data at 298K.
NH2¯ + CH3Br → CH3NH2 + Br¯
Initial Rate Experiments [ NH ¯] [ CH Br ] 2 3 M/s 1 0.10 0.10 7.5 x 10-7 2 0.20 0.20 3.0 x 10-6 3 0.20 0.40 6.0 x 10-6 a) (5) What is the rate law expression? c) (10) Would this reaction be faster or slower if the solvent was switched from DMF to CH3OH? Using reaction coordinate diagrams, explain your answer.
d) (10) Would this reaction be faster or slower if the substrate was switched to CH3I? Again, explain your answer using reaction coordinate diagrams. 6) (15) Consider the following reaction mechanism.
.....step 1..... 2 OF2 O2F4 fast, equilibrium
.....step 2..... O2F4 O2 + 2 F2 slow
a) (3) What is the overall reaction?
b) (3) What is the derived rate law expression from this mechanism?
c) (3) Plotting [OF2] vs. time is non-linear, but plotting ______vs. time would be linear.
d) (6) How long it will take for the concentration of OF2 to go from 0.25M to 0.125M at 298K if the k value is 3.92 L/mol·s?
7) (10) The The decomposition of ozone to oxygen (as shown) is catalyzed by NO gas. 2O3 3O2 Draw a reaction coordinate diagram that displays the difference between an uncatalyed decomposition reaction and a catalyzed decomposition. 8) (15) For any empty arrows, provide the nucleophile and solvent. For the empty boxes, provide the products. Make sure to draw both products for racemic mixtures and the correct orientation if it is optically active. The asterisk indicates an optically active substance.
CH2CH3
+ NaBr DMF H3C * H Cl
H2O
N3
H3C H CH2CH3
N3
H
H3C CH2CH3 Racemic Mixture
9) (5) In the reaction shown, how long does it take for the concentration
Cl + NH3 NH H2O 2
k = 0.320 s-1 a) How much time does it take for the concentration of NH3 to go from 0.050M to 0.020M. 10) (15 total) Consider the following reaction and information provided.
propene cyclopropane
-The activation energy for this reaction is 340 kJ/mol at 770K -The rate constant (k) at 773 K is 0.320 s-1. -For this reaction, the following Kc values were determined. -4 -2 Kc = 8.2 x 10 at 350 K, Kc = 1.3 x 10 at 500 K, Kc = 0.150 at 770 K a) (7) At what temperature will the rate constant be 8.25 x 10-2 s-1
b) (2) Is this reaction exothermic or endothermic? c) (2) If when at equilibrium, propene was added to the reaction, the reaction would shift to the______to reach equilibrium. d) (2) If when at equilibrium, the reaction was heated up, it would shift to the______to reach equilibrium. e) (2) If a catalyst was used to convert propene to cyclopropane, the equilibrium constant would______. 11) (10, 2 each) For each reaction set, indicate which one will have the fastest rate. Circle the letter in each to indicate your choice.
H2O A NaN3 A I DMF Br
B NaN3 H O I B 2 DMF I
NaSCH3 Br A CH3OH (solvent) NaSCH3 A CH3Cl DMSO Br B NaSCH3 B NaOCH3 CH3OH (solvent) CH3Cl DMSO
A NaBr Cl DMSO
B NaI Cl DMSO
Extra Credit (10 pts...all or nothing).
In problem 10, if the activation energy in the opposite direction is 240 kJ/mol, then what is the equilibrium constant be if the temperature was raised to 1000K? Show your work and circle your answer