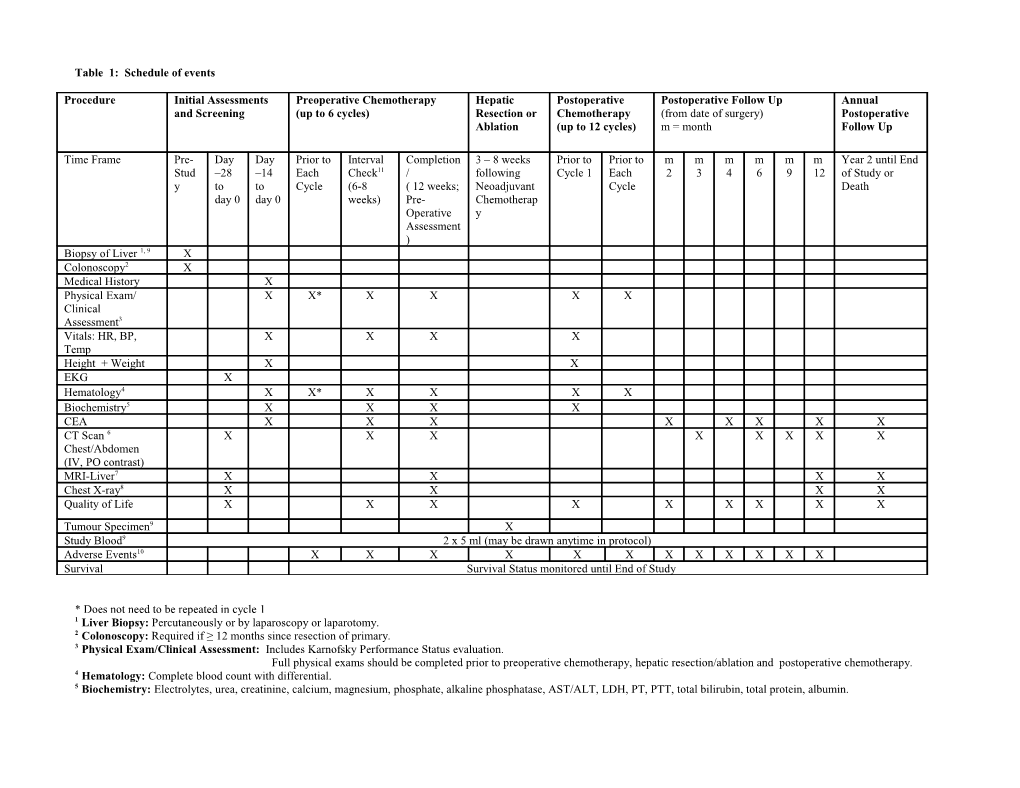
Table 1: Schedule of events
Procedure Initial Assessments Preoperative Chemotherapy Hepatic Postoperative Postoperative Follow Up Annual and Screening (up to 6 cycles) Resection or Chemotherapy (from date of surgery) Postoperative Ablation (up to 12 cycles) m = month Follow Up
Time Frame Pre- Day Day Prior to Interval Completion 3 – 8 weeks Prior to Prior to m m m m m m Year 2 until End Stud –28 –14 Each Check11 / following Cycle 1 Each 2 3 4 6 9 12 of Study or y to to Cycle (6-8 ( 12 weeks; Neoadjuvant Cycle Death day 0 day 0 weeks) Pre- Chemotherap Operative y Assessment ) Biopsy of Liver 1, 9 X Colonoscopy2 X Medical History X Physical Exam/ X X* X X X X Clinical Assessment3 Vitals: HR, BP, X X X X Temp Height + Weight X X EKG X Hematology4 X X* X X X X Biochemistry5 X X X X CEA X X X X X X X X CT Scan 6 X X X X X X X X Chest/Abdomen (IV, PO contrast) MRI-Liver7 X X X X Chest X-ray8 X X X X Quality of Life X X X X X X X X X Tumour Specimen9 X Study Blood9 2 x 5 ml (may be drawn anytime in protocol) Adverse Events10 X X X X X X X X X X X X Survival Survival Status monitored until End of Study
* Does not need to be repeated in cycle 1 1 Liver Biopsy: Percutaneously or by laparoscopy or laparotomy. 2 Colonoscopy: Required if ≥ 12 months since resection of primary. 3 Physical Exam/Clinical Assessment: Includes Karnofsky Performance Status evaluation. Full physical exams should be completed prior to preoperative chemotherapy, hepatic resection/ablation and postoperative chemotherapy. 4 Hematology: Complete blood count with differential. 5 Biochemistry: Electrolytes, urea, creatinine, calcium, magnesium, phosphate, alkaline phosphatase, AST/ALT, LDH, PT, PTT, total bilirubin, total protein, albumin. Serum pregnancy test required prior to first cycle of chemotherapy (for women of reproductive potential only). 6 CT Scans: CT of pelvis should be completed as clinically indicated (i.e. rectal cancer). If recurrent disease or if progressive to a state of non-resectability, CT scans should be completed as clinically indicated (at physician discretion). 7 MRI of Liver: Desirable at baseline and following neoadjuvant chemotherapy, but not mandatory. During Post-Operative follow up, CT and/or MRI is acceptable. 8. Chest X-ray: Required only when CT of chest is not completed. 9 Optional Specimen Collection: Additional informed consent must be given to use blood and tissue specimens for genetic testing. 10 Adverse Events: Terminology and grading based on NCI, CTCAE (Version 3.0); refer to protocol section 8.4. 11 Interval Check: If progressive disease, chemotherapy will be discontinued. Disease will be resected or ablated (if possible) and/or best supportive treatment will be offered.