Download Photographs and Batteries Were Replaced Every Five Days
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ethembeni Cultural Heritage
Phase 1 Heritage Impact Assessment Report: Proposed Rehabilitation of Regional Road R61, Section 3 Between Cradock and Tarkastad, Inxuba Yethemba and Tsolwana Local Municipalities, Chris Hani District, Eastern Cape Province, South Africa Prepared for GIBB Engineering & Science 2nd Floor, Greyville House, Cnr Greyville & Cape Rd, Greenacres, Port Elizabeth 6045 Box 63703, Greenacres 6057 Telephone Dr Norbert Klages 041 392 7500; 083 234 3399 Fax 041 363 9300 [email protected] Prepared by ETHEMBENI CULTURAL HERITAGE Elizabeth Wahl and Len van Schalkwyk Box 20057 Ashburton 3213 Pietermaritzburg Telephone 033 326 1136 / 082 655 9077 / 082 529 3656 Facsimile 086 672 8557 [email protected] 31 January 2013 Phase 1 HIA of Rehabilitation of Regional Road R61, Section 3 between Cradock and Tarkastad, Eastern Cape, South Africa MANAGEMENT SUMMARY eThembeni Cultural Heritage was appointed by GIBB Engineering & Science to undertake a Phase 1 Heritage Impact Assessment of a proposed road upgrade in the Eastern Cape Province, as required by the National Environmental Management Act 107 of 1998 as amended, in compliance with Section 38 of the National Heritage Resources Act 25 of 1999 as amended. This report represents compliance with a full Phase 1 HIA, excluding a specialist palaeontological study, which has been undertaken by Dr John Almond of Naturaviva. HERITAGE RESOURCE DESCRIPTIONS AND SIGNIFICANCE Places, buildings, structures and equipment A section of drystone walling is located directly opposite BP 1 (Dwingfontein) at the start of the road upgrade project, on the northern side of the entrance road leading to the Michau farmstead. The significance of the walling is low at all levels. -

Meandering in the Main Karoo Basin, Eastern Cape, South Africa FIELD TRIP LEADERS: Emese Bordy & Goonie Marsh
POST 10 Meandering in the main Karoo Basin, Eastern Cape, South Africa FIELD TRIP LEADERS: Emese Bordy & Goonie Marsh This comprehensive five day trip will take us through, via an unique route in the shortest travel time and distance, a geo-traverse through over 400 million years of South African geological history. The main focus of the field trip is the sedimentary fill of the southern main Karoo Basin, including the nature of some of the major Karoo intrusive and volcanic complexes of the Eastern Cape. Field Trip Leaders: Emese Bordy and Goonie Marsh Start: Port Elizabeth End: Port Elizabeth Dates: 3-8 September 2016 ITINERARY SUGGESTION OF FLIGHT BOOKING FROM CAPE TOWN TO PORT ELIZABETH: SOUTH AFRICAN AIRWAYS FLIGHT 1803 DEPARTING CAPE TOWN AT 07h00, ARRIVING PORT ELIZABETH AT 08h15 Day 1 3 September 2016, Saturday Arrival at Port Elizabeth Airport and transfer to Grahamstown Overnight at the Graham Hotel in Grahamstown BB via Addo Elephant Park Day 2 4 September 2016, Sunday Stop 1 : Overview of 400 million years of South African geological history though the rocks and landscape of Grahamstown, Eastern Cape. Topics covered: Cape and Karoo systems, formation of the main Karoo Basin and Cape Fold Belt, Gondwana breakup, Cenozoic sea level changes. Location : 1820 Settlers' Monument Features to be seen : Relationship of the geology and geomorphology Cape Fold Belt - large-scale geological and geomorphological features: Witteberg quartzite ridges with some mudstone lenses to the south (cut by the N2 national road) & to the north (called Botha’s Ridge) running across the horizon. City bowl overlying the E-W running contact between soft Witteberg mudstones to the south and Dwyka tillites to the north Flat peneplain underlain by hard silcretes (with Joza/ King’s Flat township on it), visible along the northeastern horizon. -

Land Reform, Sustainable Rural Livelihoods and Gender Relations a Case Study of Gallawater a Farm
Land reform, sustainable rural livelihoods and gender relations A case study of Gallawater A farm SCHOOLof GOVERNMENT Volume one UNIVERSITY OF THE THE WESTERN CAPE Kgopotšo Mokgope Research Report No. 5 Research Report No. 5 Land reform, sustainable rural livelihoods and gender relations: A case study of Gallawater A farm Kgopotšo Mokgope Programme for Land and Agrarian Studies November 2000 Land reform, sustainable rural livelihoods and gender relations: A case study of Gallawater A farm By Kgopotšo Mokgope Published by the Programme for Land and Agrarian Studies (PLAAS), University of the Western Cape, Private Bag X17, Bellville, 7535, telephone: 021 9593733/3961, fax: 021 9593732, email: [email protected] ISBN 1-86808-488-4 November 2000 Edited by Stephen Heyns Cover photograph by Kgopotšo Mokgope Layout by Rosie Campbell and Derek Patterson Typeset in Times Reproduction by Image Mix Printing by New Wave Contents Page List of figures ii List of tables ii Acknowledgements iii 1 Introduction: context and rationale 1 2 Key issues and concepts 14 3 Land reform, gender relations and rural livelihoods in South Africa 23 4 The history and context of Gallawater A farm 33 5 Livelihoods and livelihood resources on Gallawater A 49 6 Future prospects for Gallawater A farm 66 7 Conclusions and recommendations 73 Bibliography 81 i List of figures Figure 1: Eastern Cape location map 4 Figure 2: Magisterial districts of the Eastern Cape mentioned in this report 4 Figure 3: Farms and settlements surrounding Gallawater A 34 Figure 4: Gallawater A farm -

Rehabilitation of National Route R61 (Section 3, Km 24.2 to Km 75) Between Cradock and Tarkastad, Eastern Cape
PALAEONTOLOGICAL HERITAGE STUDY: COMBINED DESKTOP AND FIELD-BASED ASSESSMENT Rehabilitation of National Route R61 (Section 3, km 24.2 to km 75) between Cradock and Tarkastad, Eastern Cape John E. Almond PhD (Cantab.) Natura Viva cc, PO Box 12410 Mill Street, Cape Town 8010, RSA [email protected] February 2013 1. SUMMARY The South African National Roads Agency Limited (SANRAL) is proposing to rehabilitate Section 3 of the National Route R61 (km 24.2 to km 75) between Cradock and Tarkastad, Eastern Cape. The project involves widening of the roadway and of all stormwater structures along the route. Road material is to be sourced from five new or existing borrow pits and one hard rock quarry. A Phase 1 palaeontological heritage assessment for the road project has been commissioned by Arcus GIBB (Pty) Ltd in accordance with the requirements of the National Heritage Resources Act (Act 25 of 1999). Section 3 of the R61 traverses the outcrop area of continental sedimentary rocks of the Upper Beaufort Group (Tarkastad Subgroup, Karoo Supergroup) of Early to Middle Triassic age. These are cut and baked by numerous dolerite intrusions of the Karoo Dolerite Suite of Early Jurassic age. Towards Cradock (Graaff-Reinet and Middelburg 1: 250 000 sheet areas) the sedimentary bedrocks belong to the sandstone-dominated Katberg Formation that was deposited in arid braided fluvial settings following the catastrophic end-Permian mass extinction event. Further east towards Tarkastad (Queenstown and King William’s Town 1: 250 000 sheet areas) the sedimentary bedrocks are assigned to the slightly younger Burgersdorp Formation comprising recessive-weathering reddish mudrocks and braided river channel sandstones. -
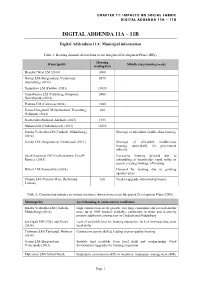
Digital Addenda 11A – 11B
CHAPTER 11: IMPACTS ON SOCIAL FABRIC DIGITAL ADDENDA 11A – 11B DIGITAL ADDENDA 11A – 11B Digital Addendum 11A: Municipal information Table 1: Housing demand, drawn from recent Integrated Development Plans (IDPs). Housing Municipality Middle class housing needs waiting lists Beaufort West LM (2014) 3000 Gariep LM (Burgersdorp, Venterstad, 4570 Steynsburg) (2013) Ngqushwa LM (Peddie) (2013) 10320 Umsobomvu LM (Colesberg, Noupoort, 2000 Norvalspont) (2014) Hantam LM (Calvinia) (2014) 1000 Karoo-Hoogland LM (Sutherland, Fraserburg, 880 Williston) (2014) Nxuba LM (Bedford, Adelaide (2013) 1923 Makana LM (Grahamstown) (2014) 16582 Inxuba Yethemba LM (Cradock, Middelburg) Shortage of affordable middle-class housing (2014) Gariep LM (Burgersdorp, Venterstad) (2011) Shortage of affordable middle-class housing, particularly for government officials Sarah Baartman DM (Grahamstown, Graaff- Increasing housing demand due to Reinet) (2015) unbundling of households; rapid influx of people creating shortage of housing Ikwezi LM (Jansenville) (2014) Demand for housing due to growing squatter areas Ubuntu LM (Victoria West, Richmond, 820 Need to upgrade old township houses Loxton) Table 2: Construction industry in various localities, drawn from recent Integrated Development Plans (IDPs). Municipality Local housing & construction conditions Inxuba Yethemba LM (Cradock, High construction sector growth; two large companies and several smaller Middelburg) (2014) ones; up to 1000 workers available; constraints in water and electricity prevent additional construction in Cradock and Middelburg Joe Gqabi DM (HQ Lady Grey) Lack of available land for housing expansion; lack of serviced sites; poor (2014) local skills Tsolwana LM (Tarkastad, Hofmeyr Contractors poorly skilled, leading to poor quality housing (2014) Gariep LM (Burgersdorp, Suitable land available. Poor local skills and workmanship. Need Venterstad) (2013) infrastructure upgrades for housing expansion Maletswai LM (Aliwal North, Inadequate construction skills in localities; inadequate management skills. -

SFD Promotion Initiative Tarkastad
SFD Promotion Initiative Tarkastad Enoch Mgijima Local Municipality, Chris Hani District Municipality Eastern Cape, South Africa SFD Lite Final Report This SFD Lite Report was created through field-based research by Emanti Management and Centre for Science and Environment for a Water Research Commission project and as part of the SFD Promotion Initiative. Date of production: March 2019 Last update: March 2019 Tarkastad Produced by: Executive Summay South Africa EMANTI SFD Lite Report The SFD Promotion Initiative (SFD PI) has developed recommended methods and tools for preparing SFD Graphics and Reports. A full SFD Report consists of the SFD Graphic, the analysis of the service delivery context and enabling environment for service provision in the city for which you are preparing your SFD, and the complete record of data sources used. This analysis allows a systemic understanding of excreta management in the city, with evidence to support it. As a starting point (first step stone) to this (explained in detail in the SFD Manual), the SFD Lite is a simplified reporting template that summarises the key information about the excreta management situation in the city. SFD Lite Report Tarkastad, South Africa, 2018 Produced by: Thabisa Manxodidi, Emanti Unathi Jack, Emanti ©Copyright The tools and methods for SFD production were developed by the SFD Promotion Initiative and are available from: www.sfd.susana.org. All SFD materials are freely available following the open-source concept for capacity development and non-profit use, so long as proper acknowledgement of the source is made when used. Users should always give credit in citations to the original author, source and copyright holder. -

Chris Hani District Municipality 2
Office of the Municipal Manager Supply Chain Management TEL: 045 808 4662 /63 /54 /55 PRIVATE BAG X 7121 FAX: 045 839 2437 QUEENSTOWN, 5320 Email: [email protected] Enq: Date: 29 October 2014 To: Andile Feleni Please supply us with your bank details in your quotation. Please Fax them to this number 045 839 2437. NO QUAN DESCRIPTION TITY 1. 9 Office Signage Boards: To be installed in Inxuba Yethemba and Tsolwana municipalities. This will include a standard design; printed in full colour. Content will be provided. NB: To be mounted on 2 poles, at least 30cm circumference or welded on a wall (no poles will be needed in this case). Size: : 2,5 x 1,5 meters Areas are as follows: 1. Inxuba Yethemba Local Municipality: Cradock No 09 Beeren Street Cradock 5880 2. Inxuba Yethemba Local Municipality: Cradock No 05 Sikulu Street Lingelihle Cradock 5880 3. Inxuba Yethemba Local Municipality: Cradock Erf No. 2132 Main road/Hoofweg Michausdal Cradock 5880 4. Inxuba Yethemba Local Municipality: Middelburg Kwa-Nomzame Rent Office Site No. 3211 Cety8we Street Middelburg 5900 5. Inxuba Yethemba Local Municipality: Middelburg Midros Community Hall Site No.1172 Miles Road Middelburg 5900 6. Inxuba Yethemba Local Municipality: Middelburg Inxuba Yethemba Local Municipality – Technical Services Building Site No.1864 New Street Middelburg 5900 7. Inkwanca Local Municipality: Molteno 423 Stewart Street Molteno 5500 8. Inkwanca Local Municipality: Sterkstroom 57 John Vorster Drive Sterkstroom 5425 9. Tsolwana Local Municipality: Tarkastad No. 1 Butekant Street Hofmeyer 5930 10. Tsolwana Local Municipality: Hofmeyer No. 196 Molteno Street Hofmeyer 5930 Please note: Signage to be installed by service provider Service provider to bring own installation materials i.e. -

Palaeontological Field Assessment for the Proposed Helpmekaar Dam & Pipeline (Ribbokkop Boerdery) Near Tarkastad in the Eastern Cape
PALAEONTOLOGICAL FIELD ASSESSMENT FOR THE PROPOSED HELPMEKAAR DAM & PIPELINE (RIBBOKKOP BOERDERY) NEAR TARKASTAD IN THE EASTERN CAPE Compiled for: isi-Xwiba Consulting CC PO Box 2097, Komani 5322 [email protected] Prepared by Banzai Environmental 12 December 2020 Declaration of Independence I, Elize Butler, declare that – General declaration: • I act as the independent palaeontological specialist in this application • I will perform the work relating to the application in an objective manner, even if this results in views and findings that are not favorable to the applicant • I declare that there are no circumstances that may compromise my objectivity in performing such work; • I have expertise in conducting palaeontological impact assessments, including knowledge of the Act, Regulations and any guidelines that have relevance to the proposed activity; • I will comply with the Act, Regulations and all other applicable legislation; • I will take into account, to the extent possible, the matters listed in section 38 of the NHRA when preparing the application and any report relating to the application; • I have no, and will not engage in, conflicting interests in the undertaking of the activity; • I undertake to disclose to the applicant and the competent authority all material information in my possession that reasonably has or may have the potential of influencing - any decision to be taken with respect to the application by the competent authority; and - the objectivity of any report, plan or document to be prepared by myself for submission -

Situation Assessment for the Middle Kei Sub-Area
6.1 6. SITUATION ASSESSMENT OF THE MIDDLE KEI SUB - AREA 6.1 General Overview 6.1.1 Topography and Rivers This sub-area consists of the middle reaches of the Great Kei River and two main tributaries, the Tsomo River with its headwaters in the Stormberg (S50), and the Thomas River with its headwaters in the Amatola mountain range (S40). Both rivers are regulated with the Ncora and Tsojana Dams in the Tsomo River catchment and the Sam Meyer Dam in the Thomas River catchment. Figure 6.1 The Middle Kei Sub-area The Middle Kei sub-area lies predominantly within the inland plateau topographical zone. The Tsomo River valley has its source in the southern Drakensberg range (the Stormberg) at an altitude of approximately 2 500 masl and then descends in a fairly steep valley to enter the Great Kei River at 850 masl. The Thomas River has its source on the north eastern slopes of the Amatola mountain range at an altitude of approximately 1 800 masl before flowing in an easterly direction to join the Great Kei River at 1 000 masl. 6.1.2 Climate and Rainfall The climate in this sub-area shows a similar pattern to that of the Upper Kei sub-area with hot and dry summer months to very cold winter months i.e. temperatures of over 6.2 40o C in summer and very cold (-10o C) in winter with widespread frost and snowfalls on the high lying ground. There is a high temporal and spatial variation in rainfall over the catchment with the Mean Annual Precipitation (MAP) varying from approximately 600 mm in the Great Kei valley to a maximum of 1 200 mm in the Amatola and Stormberg mountains in the east and north respectively. -

Phase 1 Archaeological Impact Assessment Of
Palaeontological Impact Assessment of the Cluster 9 Bulk Water supply pipeline at Tsomo, Chris Hani District Municipality, EC Province. Report prepared for: isi-Xwiba Consulting By Lloyd Rossouw PO Box 38806 Langenhovenpark Executive Summary • Several sections of the proposed pipeline as well as the water extraction facility are located on potentially fossil-bearing Tarkastad Subgroup (Trlk, Trlb) strata. • The overlying Quaternary deposits bear little palaeontological significance. • The intrusive dolerites bear little palaeontological significance. • There are no major palaeontological grounds to suspend the proposed development of the site. • However, any developments that may destroy or damage subsurface fossils are of conservation and research interest. • In such a case it is advised that newly uncovered material found during the course of excavation activities within footprint must be reported to SAHRHA, that excavations into in situ sedimentary bedrock should allow for inspection by a specialist at the appropriate time and that possible intact finds may require a Phase 2 rescue operation at the cost of the developer. 2 Contents Executive Summary ....................................................................................................... 2 Introduction .................................................................................................................... 4 Terms of reference ................................................................................................. 4 Description of the Affected Area -

Accredited COVID-19 Vaccination Sites Eastern Cape
Accredited COVID-19 Vaccination Sites Eastern Cape Permit Primary Name Address Number 202103960 Fonteine Park Apteek 115 Da Gama Rd, Ferreira Town, Jeffreys Bay Sarah Baartman DM Eastern Cape 202103949 Mqhele Clinic Mpakama, Mqhele Location Elliotdale Amathole DM Eastern Cape 202103754 Masincedane Clinic Lukhanyisweni Location Amathole DM Eastern Cape 202103840 ISUZU STRUANWAY OCCUPATIONAL N Mandela Bay MM CLINIC Eastern Cape 202103753 Glenmore Clinic Glenmore Clinic Glenmore Location Peddie Amathole DM Eastern Cape 202103725 Pricesdale Clinic Mbekweni Village Whittlesea C Hani DM Eastern Cape 202103724 Lubisi Clinic Po Southeville A/A Lubisi C Hani DM Eastern Cape 202103721 Eureka Clinic 1228 Angelier Street 9744 Joe Gqabi DM Eastern Cape 202103586 Bengu Clinic Bengu Lady Frere (Emalahleni) C Hani DM Eastern Cape 202103588 ISUZU PENSIONERS KEMPSTON ROAD N Mandela Bay MM Eastern Cape 202103584 Mhlanga Clinic Mlhaya Cliwe St Augustine Jss C Hani DM Eastern Cape 202103658 Westering Medicross 541 Cape Road, Linton Grange, Port Elizabeth N Mandela Bay MM Eastern Cape Updated: 30/06/2021 202103581 Tsengiwe Clinic Next To Tsengiwe J.P.S C Hani DM Eastern Cape 202103571 Askeaton Clinic Next To B.B. Mdledle J.S.School Askeaton C Hani DM Eastern Cape 202103433 Qitsi Clinic Mdibaniso Aa, Qitsi Cofimvaba C Hani DM Eastern Cape 202103227 Punzana Clinic Tildin Lp School Tildin Location Peddie Amathole DM Eastern Cape 202103186 Nkanga Clinic Nkanga Clinic Nkanga Aa Libode O Tambo DM Eastern Cape 202103214 Lotana Clinic Next To Lotana Clinic Lotana -

Provincial Gazette / Igazethi Yephondo / Provinsiale Koerant
PROVINCE OF THE EASTERN CAPE IPHONDO LEMPUMA KOLONI PROVINSIE VAN DIE OOS-KAAP Provincial Gazette / Igazethi Yephondo / Provinsiale Koerant BHISHO/KING WILLIAM’S TOWN PROCLAMATION by the MEC for Economic Development, Environmental Affairs and Tourism January 2020 1. I, Mlungisi Mvoko, Member of the Executive Council (MEC) for Economic Development, Environmental Affairs and Tourism (DEDEAT), acting in terms of Sections 78 and 79 of the Nature and Environmental Conservation Ordinance, 1974 (Ordinance No. 19 of 1974), and Section 18 of the Problem Animal Control Ordinance, 1957 (Ordinance 26 of 1957) hereby determine for the year 2020 the hunting season and the daily bag limits, as set out in the second and third columns, respectively, of Schedule 1, hereto in the Magisterial Districts of the Province of the Eastern Cape of the former Province of the Cape of Good Hope and in respect of wild animals mentioned in the first column of the said Schedule 1, and I hereby suspend and set conditions pertaining to the enforcement of Sections 29 and 33 of the said Ordinance to the extent specified in the fourth column of the said Schedule 1, in the district and in respect of the species of wild animals and for the periods of the year 2020 indicated opposite any such suspension and/or condition, of the said Schedule 1. 2. In terms of Section 29 (e), [during the period between one hour after sunset on any day and one hour before sunrise on the following day], subject to the provisions of this ordinance, I prohibit hunting at night under the following proviso, that anyone intending to hunt at night for management purposes by culling any of the Alien and Invasive listed species, Hares, specified species, Rodents, Porcupine, Springhare or hunting Black-backed jackal, Bushpig and Caracal, in accordance with the Ordinance, must apply to DEDEAT for a provincial permit and must further notify the relevant DEDEAT office, and where applicable the SAPS Stock Theft Unit, during office hours, prior to such intended hunt.