Name: ______Leveled , υ & Energy Calculation Practice
Equations & constants C = 2.998 x 108 m/s H = 6.626 x 10-34 Js c =υ E = hυ
These homework problems will help you test your own knowledge of the material above. They are leveled and get increasingly harder. Show all work for credit! Level 1 1) If a wave has a frequency of 9.05 x 1015 Hz; calculate its wavelength in nm.
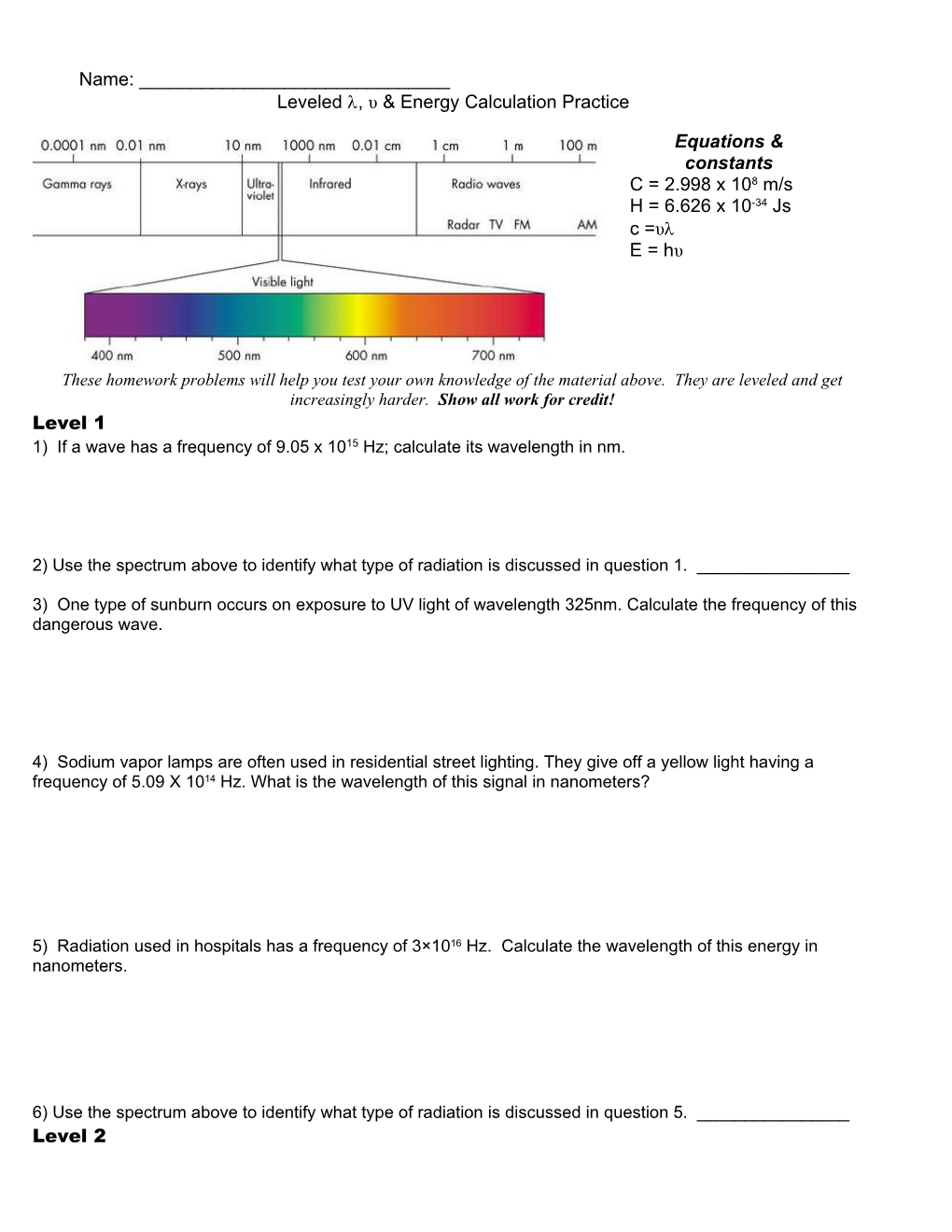
2) Use the spectrum above to identify what type of radiation is discussed in question 1. ______
3) One type of sunburn occurs on exposure to UV light of wavelength 325nm. Calculate the frequency of this dangerous wave.
4) Sodium vapor lamps are often used in residential street lighting. They give off a yellow light having a frequency of 5.09 X 1014 Hz. What is the wavelength of this signal in nanometers?
5) Radiation used in hospitals has a frequency of 3×1016 Hz. Calculate the wavelength of this energy in nanometers.
6) Use the spectrum above to identify what type of radiation is discussed in question 5. ______Level 2 7) From question #3; burning radiation from the sun has a wavelength 325nm. What is the energy of a photon of this wavelength?
8) Calculate the energy of waves from an AM radio station that has a frequency of 1210 kHz (that’s kilohertz, so you must convert to hertz to get correct answer).
9) It requires a photon with a minimum energy of 4.41 x 10-19J to emit electrons from sodium metal. What is the minimum frequency of light necessary to emit electrons (In Hz)?
Level 3 10a) What wavelength (in nm)of radiation has photons of energy 2.87 x 10-18J?
10b)In what portion of the electromagnetic spectrum would this radiation be found? ______
11) Sun tan lotion does the job of protecting us from UV-B waves that have a wavelength of approximately 280nm. What is the frequency and energy associated with those waves?