Photoinduced Electron Transfer Reactions of Thiobenzoates
Introduction and Background
Irradiation of thiobenzoates can produce alkenes by way of a Norrish Type II process. Barton and others have studied the synthetic applications of the photochemical -hydrogen abstraction of thiobenzoates.1-6 The elimination of thiobenzoic acid to form an alkene is stereo- and regioselective for the axial allylic hydrogen in the cholesterol derivative 1 (Figure 1).1 Likewise, photolysis of 2-ethoxyethyl thiobenzoate (2) gives ethyl vinyl ether in 46% yield in less than one hour.4
Figure 1. Alkene Formation from Thiobenzoates Me Me C8H17 Me C H (D)H Me 8 17 h O Ph C D(H) D(H) S 1 O
OEt + Ph C SH O h OEt C S 2
While the elimination occurs with compounds possessing a -bond or heteroatom at the - carbon, it fails with saturated hydrocarbons. The success of the reaction depends on the strength of the -C-H bond. For example, the quantum yields for the disappearance of Ph(C=S)OCH2CH2R are 0.46, 0.12 and 0.01 when R is p-MeOPh, EtO and Me, respectively. The corresponding bond -C-H bond energies are 81, 91 and 96 kcal/mol. Thus, an -alkoxy group only weakly activates the reaction.6
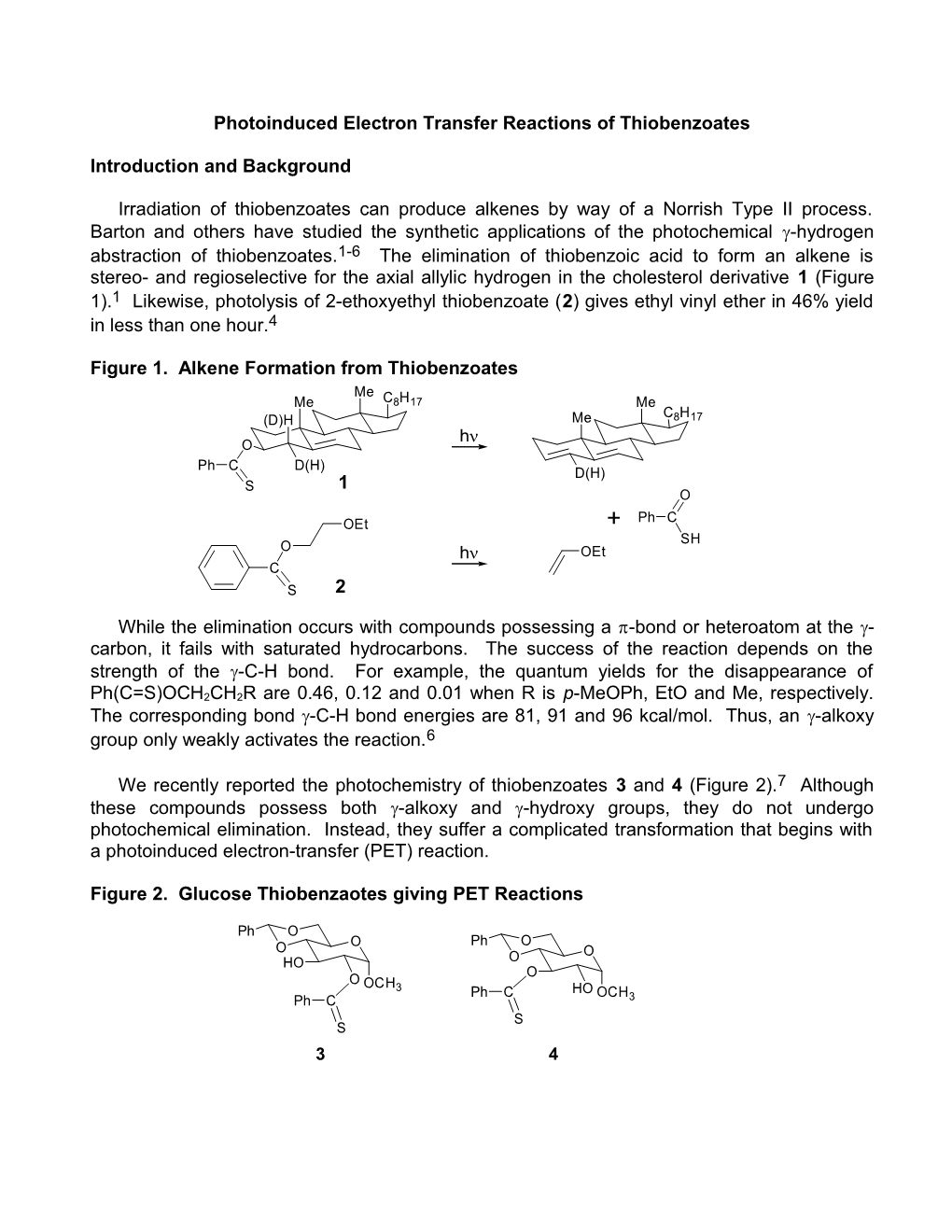
We recently reported the photochemistry of thiobenzoates 3 and 4 (Figure 2).7 Although these compounds possess both -alkoxy and -hydroxy groups, they do not undergo photochemical elimination. Instead, they suffer a complicated transformation that begins with a photoinduced electron-transfer (PET) reaction.
Figure 2. Glucose Thiobenzaotes giving PET Reactions
Ph O O Ph O O O HO O O O OCH 3 Ph C HO OCH Ph C 3 S S 3 4 Irradiation of 3 and 4 in CH2Cl2 in the presence of NEt3 results in nearly quantitative product formation within 15 min. Each thiobenzoate gives two main products that are assigned as 5a,b and 6a,b, respectively (Figure 3).
Figure 3. Products from the PET Reactions of 3 and 4
Ph O Ph O O O O O O OCH 3 O OCH3 O R S 2 O R 1 S R1 R2
5a, R1 = H, R2 = Ph 6a, R1 = Ph, R2 = H 5b, R1 = Ph, R2 = H 6b, R1 = H, R2 = Ph
We proposed that these products arise by a mechanism that involves a photoinduced electron-transfer step (Scheme 1).8-11 Irradiation of the thiobenzoate rapidly produces the excited n* triplet state through intersystem crossing. The quantum yield for triplet state formation is nearly unity. The excited triplet-state abstracts an electron from NEt 3. The resulting radical anion reacts with the solvent by nucleophilic substitution to generate a chloromethyl sulfide benzyl radical. The radical abstracts a hydrogen atom from the triethylamine radical cation. In the presence of NEt3, the chain cyclizes at the adjacent hydroxyl group.
Several experiments with deuterium-labeled solvents and reagents helped elucidate the mechanism. Irradiation in CD2Cl2 gives complete loss of the signals assigned to the diastereotopic CH2 groups. Irradiation using NEt3-d15 leads to partial loss of the thiobenzylic resonances (65% and 66% loss for 3 and 4, resp.). Finally, with 3- and 4-D (exchanged using MeOD) in CH2Cl2 using NEt3, irradiation also leads to partial loss of the thiobenzylic resonances (27% and 31% loss for 3 and 4, resp.).
Scheme 1. PET Mechanism for the Formation of 5 and 6
3* H S 1) h S NEt3 S H NEt3 + Cl C Ph O 2) isc Ph O Ph O Cl
NEt2 CH2Cl CH2Cl S NEt2 CH HC H S H + 3 O Ph H3CCH Ph O
Cl O CH2 OH NEt3 S S + HNEt3 Cl H H Ph O Ph O
2 The electron-transfer mechanism is supported by several observations. Most revealing is that the reaction occurs only with added NEt3. Cyclic voltammetry studies on 3 and 4 give + reduction potentials of -1.56 and -1.57 V in CH3CN, respectively, vs. Ag/Ag . According to the 0-0 Weller equation (Equation 1) and using a value of 2.75 eV for ET the proposed electron- transfer reaction is exothermic by 0.24 eV.
G E(D / D) E(A / A) E(0 0) E(coul) E(solv) (1)
The failure of photoexcited 3 and 4 to undergo -H abstraction is likely due to constraints imposed by the fused ring systems. The added activation energy counteracts the weakly activating effects of the -alkoxy or -hydroxy groups.
The photochemistry of the thiocarbonyl group has been documented in a number of reviews.12-14 Besides the Norrish Type II process, photoexcited thiocarbonyls can dimerize, oxidize, undergo cycloadditions and suffer cleavage reactions. The excited thiocarbonyl group has rarely been implicated in electron transfer reactions. In fact, one review goes so far as to 1 3 claim that "the excited thiones in both the S2 (, *) and the lowest (n,*) states seem to react with donor or acceptor molecules with a small contribution from charge transter".15 Recent studies of pyridinethione, 6H-purine-6-thione, and N-hydroxyacridine-9-thione have shown that their excited triplet states are reduced by tetrmethylbenzidine, but no subsequent reactions were reported.16-18
This proposal asks for support to investigate the mechanism, scope and synthetic applications of electron-transfer reactions of thiobenzoates. The justification for the study is that this type of reactivity of thiocarbonyl compounds is new, unusual, and may lead to useful synthetic methods. The PET reactions are unusual in that they differ dramatically from oxygen analogues. Excited carbonyls suffer photoreduction with amines. The ketyl anions react by protonating the negatively charged oxygen. With 3 and 4 the negatively charged sulfur reacts by nucleophilic substitution and not by protonation. Transformations involving sulfur are important for the synthesis pencillins, thiosugars and peptides.19,20 Proposed Research
1. Mechanistic Investigations
(a) Test Substrate and Delineation of the H-abstraction/PET Borderline
The glucose thiobenzoates are acid-sensitive, and are poor substrates for mechanistic investigations. We propose to use several alternative substrates to validate the electron- transfer mechanism (Figure 4). These test molecules we will also probe the borderline between PET and the -H abstraction processes.
Figure 4. Thiobenzoate Substrates OH OH S Ar = Ph, p-NO2Ph, p-CH3OPh O S O OH S O Ar Ar Ar 3 7 8 9 Compound 7 is derived from trans-1,2-cyclohexanediol. Of the three, it most nearly resembles the glucose compounds. If the PET transfer pathway is not competitive here, then we will have established that the cyclohexane ring is flexible enough for the H-abstraction pathway and that it is the benzylidene ring that shuts down the H-abstraction pathway in the glucose compounds. The second substrate (8) comes from cis-1,2-cyclohexanediol. The equatorial -H should disfavor the H-abstraction route, but it may also affect the cyclization with methylene chloride. The last substrate (9) is a derivative of a vicinal trans-diol. The bicyclic ring structure should be inflexible enough to prevent -H abstraction. One of these substrates will be chosen to study the PET process in detail. The substrate will be irradiated in methylene chloride in the presence of amine or other electron donor. The extent of the reaction will be monitored by loss of the characteristic yellow absorption of the thiobenzoate chromophore.
(b) Dependence on Donor Oxidation Potential
We have established that triethylamine is necessary for the formation of the unusual products. A reaction that proceeds by an electron-transfer pathway will be dependent on the oxidation potentials of the donor (vide supra). Electron-transfer will occur rapidly for reagents with a sufficiently low oxidation potential, but not with those with higher potentials. We will observe the dependence of the reaction progress with the following amine donors (Table 1). Each amine has a higher oxidation potential than triethylamine.21,22 PET should be endothermic for those amines with an oxidation potential greater than 1.0 V. A number of non- amine donors will also be tested. In the choice of alternative donors, it is important to make sure that the donor's triplet energy is higher that that of the thiobenzoate. Otherwise, the donor will quench the excited triplet thiobenzoate by energy transfer, not electron transfer. The naphthalene compound is borderline in this requirement, but the triplet-state of the trimethoxybenzene is out of the range of the thiobenzoate.
Table 1. Amine and Other Donors Designed to Corroborate the PET Mechanism O N N amine N N N N N
. E(D+ /D) vs. SCE 0.76 0.81 0.83 0.96 0.99 1.20
OCH3 OCH3 OCH other 3 donors
OCH3 OCH3 . E(D+ /D) 1.10 1.12 vs. SCE
4 (c) Dependence on Thiobenzoate Reduction Potential
The electron-transfer reaction depends on both the oxidation potential of the donor and the excited state reduction potential of the thiobenzoate. The presence of the benzene moiety allows for the modulation of the reduction potential by the introduction of substituents. We will examine two extreme cases: a nitro substituent and a methoxy substituent. The same series of amines will be used as the electron donors. If the PET hypothesis is correct, then the cutoff will occur at a lower oxidation potential with the nitrophenyl Ar group and at a higher oxidation potential with the methoxyphenyl Ar group. If a H-abstraction/PET borderline is established in (a), then the nitrophenyl and methoxyphenyl substrates will be examined to see if the substituents can affect the mechanistic pathway. Barton has shown that these substituents do not significantly affect the H-abstraction pathway in O-phenethyl thiobenzoates, although the reaction is slightly slower with the nitrophenyl group.3
(d) Solvent Effects
The formation of an ion pair can be controlled to some extent by solvent polarity. It is unusual to have ion pairs form in methylene chloride since this solvent is not very polar ( = 9.1). The photochemical H-abstraction reactions cited above were often conducted in cyclohexane ( = 2.0) where the formation of ion pairs would not be energetically feasible. We will conduct irradiations in solvents of different polarity using donor-substrate pairs that are on the H-abstraction/PET borderline. The PET pathway should be made more favorable as more polar solvents are selected. Suitable solvents would be acetone ( = 20.7) and acetonitrile ( = 37.5)
(e) H-atom Source
Triethylamine is the major source of the benzylic hydrogen atom. We will explore what happens when that source is removed and/or modified. The alternate amine donors are shown in Table 2.
Table 2. Alternate amine donors. C-H C-H N-H
H3C CH2 N H Y Y Y
H3C CH2
H3C N CH3 Y N N H3C
H3C N H Y N Y
H3C
Ph N Ph N N N Ph
5 Ph N N Y N H Ph The secondary amines (diethyl, dimethyl and diphenyl) allow for the possibility of proton transfer from the amino group in analogy with the reaction of singlet stilbenes.23 Proton transfer could result in the photoreduction of the thiocarbonyl group. Triphenylamine is a tertiary amine without -C-H bonds. It will reveal what happens in the reaction when the radical is denied a facile a H-atom source. Radical coupling may be ultimate result. Finally, trimethylamine offers an -C-H, but will produce an iminium cation that may react with a thiocarbonyl radical anion, but only if H-atom abstraction preceeds nucleophilic attack on methylene chloride.
(f) CIDNP Studies
The formation of a geminate triplet radical ion pair by photoinduced electron-transfer from the triplet excited state of the thiobenzoate should give rise to net CIDNP effects.24 There are several steps that must occur to account for the product formation, but the order in which they occur is an educated guess. CIDNP studies are ideally suited to answer these mechanistic questions. The thiobenzoate radical ion should have a large g-factor that the amino radical cation due to the sulfur. Significant hyperfine coupling should be present with the aryl protons (- hfc) and the protons next to the nitrogen in the donor amine (+ hfc). The first question that CIDNP can address is the reaction efficiency. If polarized amine and thiobenzoate are observed, it means that the initial triplet radical ion pair is slow to react, at least slow enough to allow for intersystem crossing to the singlet radical ion pair, which then suffers reverse electron-transfer to regenerate the reactants (Scheme 2).
Scheme 2. Potential Radical Pairs giving rise to CIDNP Effects.
proton 3 H C 3 1 SH 3 transfer S S back e- S R C NEt2 R NEt R R + Ar O 3 NEt3 transfer NEt3 Ar O H Ar O Ar O H atom cage escape transfer
S H3C 1 H C SH 3 S R C NEt Ar O + 2 C NEt2 R R H H Ar O H Ar O CH2Cl2 H atom H atom CH2Cl2 transfer transfer H2 CH2Cl CH2Cl C O S S S H C S H-atom SH 2 R R + R + NEt Ar O Ar O R C NEt2 Ar O 3 source Ar O Ar O H H H H
The CIDNP effects can provide insight into a number of other possible steps. For example, the final product can arise from either the singlet (recombination) manifold or the triplet (escape) manifold. The signs of the observed effects will indicate which pathway is operating.
6 If the product derives from the triplet manifold, CIDNP effects will provide some indication of how fast the thiocarbonyl radical anion attacks methylene chloride. If nucleophilic attack is slow, then substitution will occur after escape from the initial radical ion pair as shown in Scheme 2. The methylene protons should not show any polarization. On the other hand, a fast reaction with methylene chloride could lead to substitution of the initial radical ion pair with a radical/radical cation pair. In this case the methylene protons should show some polarization. Finally, in analogy with carbonyl photochemistry, the radical ion pair could react by proton transfer to give a thiobenzyl/--aminoethyl radical pair. This pathway would be indicated by the polarization of the methyl groups in triethylamine.25,26 We did not determine the fate of the triethylamine in our previous study. CIDNP effects should reveal into which compound it transforms.
2. Reaction Scope and Synthetic Applications
(a) Variation of the Electrophile
Methylene chloride is a convenient solvent and reactant. It offers two leaving groups, but in our mechanism only one of these reacts with the photogenerated thiocarbonyl radical anion. We will conduct the PET reactions in inert solvents that contain other possible electrophiles (Figure 5). Figure 5. PET Reactions with other Electrophiles
OH OH h 7 + CH3CH2 Cl O NEt3 O CH3CN S Ar S Ar
Ar OH O S h 10 + CHCl3 O S O Ar NEt3 O OH
OH O Cl Cl h 7 + CH CH 2 2 S O NEt 3 O CH3CN S Ar Ar
For example, chloroethane as the electrophile only allows for one nucleophilic displacement with 7. The cyclization that occurs with 3 and 4 is not possible here. The product itself isn't particularly interesting, but it will show that the second displacement is not a critical part of the PET mechanism. The reaction of photoexcited 10 with chloroform is arguably a stretch. The three chlorine atoms will retard the nucleophilic substitution by an SN2 process, and the double cyclization will be difficult. The reaction of photoexcited 7 with 1,2- dichloroethane will measure of the facility of the second cyclization. In the reaction with 3 and
7 4, a chloromethyl sulfide reacts with a hydroxyl group to form a seven-membered ring. The sulfur likely assists the chloride leaving, making way for attack by the neutral oxygen. In the case here, a 2-chloroethyl sulfide will form. The sulfur can still participate in the cyclization by displacing the chloride to form an episulfonium ring. This reaction would result in the formation of an eight-membered ring.
(b) Intramolecular Nucleophilic Substitution
In our previous work the thiobenzoate radical anion reacts with methylene chloride to form a chloromethyl group. In subsequent steps a remote hydroxyl group reacts with this group to form a seven-membered ring. We will explore whether the reduced thiobenzoate group can displace intramolecular leaving groups. These displacements are similar to classic neighboring group participation reaction; however, the bridged intermediate is trapped in this case. The series of substrates and potential products is shown in Figure 6.
Figure 6. Thiobenzoate Neighboring Group Effects Br Br h S H 11 Ph h S Ph 13 NEt O C O 3 O Ph NEt H S 3 O S Ph
Br Br h S h S Ph Ph NEt 12 14 3 H O C H NEt3 O S O O S Ph Ph Compound 11 is the simplest substrate bearing an internal bromine leaving group. Since the strength of the (Br)C-H bond in CH3Br is several kcal higher than the (CH3O)C-H in 27 CH3OCH3, the possibility of H-abstraction is remote. The CH3CBr2-H bond strength has been experimentally determined as 94.9 kcal/mol.28 We will use information from the mechanistic studies to maximize the PET process by choosing the optimal aryl group and solvent. The product is a benzylidene thioacetal, a 1,3-oxathiolane. The benzylidene group can be removed by acid hydrolysis to liberate the vicinal mono-thioglycol.29 The H-abstraction mechanism is not possible in 12. The product is also a benzylidene thioacetal, but instead a 1,3-oxathiane. Deprotection generates a 1,3-mercaptoalcohol. The next two substrates are ring analogues of 11 and 12. Compound 13 should give a cis-thioglycol. In compound 14 the reaction requires the thiobenzoate group to be axial and the leaving group equatorial. The latter requirement may the cyclization problematic.
If these substrates prove viable, they may also be used in the mechanistic studies.
(c) Ramburg-Bäcklund Reaction: Formation of Aryl Vinyl Ethers
The chloromethyl sulfide intermediate formed as a result of the PET reaction seems a convenient precursor for the Rämburg-Backlund reaction of -halo sulfones.30-32 If the second hydroxyl group of our thiobenzoates is removed, as in 15, then it may be possible to
8 isolate chloromethyl sulfide intermediate (Figure 7). Oxidation to the sulfone followed by treatment with base should produce the aryl vinyl ether.
Figure 7. Ramburg-Bäcklund Reaction
h + CH2Cl2 O NEt3 O 15 S Ar S Ar
CH2Cl
MCPBA
NaOCH3
CH OH O 3 O
H2C Ar O2S Ar
CH2Cl
(d) Internal Amine Donor: C-H Substitution
Placing the amine donor as part of the thiobenzoate may promote an alternative reaction course (Scheme 3). The enforced proximity of the benzylic radical to the -amino C-H may promote H-atom abstraction. The thiolate ion should react readily with the iminium ion resulting in net substitution of the H with S.
Scheme 3. C-H Substitution with an Internal Amine Donor
Ar S H h O O N S N CH3CN Ar 16
Ar Ar S S H H N O O N
(e) Internal Radical Traps
The radical portion of the thiocarbonyl radical anion could react with an accessible -bond. A 1,5 exo-trig cyclization would be optimal. Three substrates satisfying this description are shown in Figure 8.
9 Figure 8. Thiobenzoates with Internal Radical Traps
S O h O S Ar NEt3 Ar CH3CH2Cl CH3CN 17
S O O Ar h Ar S
CH3CN
NMe2 NMe2 18
Ar h S Ph O S NEt3 Ph O Br CH3CN Ar 19
Compounds 17 and 18 have norbornane skeletons to avoid the Norrish II process which is efficient with allylic C-H bonds. Compound 17 offers a simple alkene trap, whereas in compound 18 the trap is also the electron donor. The overall process with 18 is an internal [2+2] cycloaddition. In compound 19 the internal nucleophilic displacement of the bromine is followed by an 1,6 exo-trig cyclization.
10 References Cited
1. Achmatowicz, S.; Barton, D. H. R.; Magnus, P. D.; Poulton, G. A.; West, P. J., "Photochemical Transformations. Part XXX. Photolysis of Thiobenzoic Acid O-Esters. Part I. Photolysis of O-Cholesteryl Thiobenzoate," J. Chem. Soc. Perkin Trans. I 1973, 1567- 1570.
2. Barton, D. H. R.; Chavis, C.; Kaloustian, M. K.; Magnus, P. D.; Poulton, G. A.; West, P. J., "Photochemical Transformations. Part XXXI. Photolysis of Thiobenzoic Acid O-Esters. Part II. General Methods for the Preparation of Thiobenzoic Acid O-Esters," J. Chem. Soc. Perkin Trans. I 1973, 1571-1574.
3. Barton, D. H. R.; Bolton, M.; Magnus, P. D.; Marathe, K. G.; Poulton, G. A.; West, P. J., "Photochemical Transformations. Part XXXII. Photolysis of Thiobenzoic Acid O-Esters. Part III. Photolysis of O-Phenethyl Thiobenzoates and Other Thiobenzoates," J. Chem. Soc. Perkin Trans. I 1973, 1574-1579.
4. Barton, D. H. R.; Bolton, M.; Magnus, P. D.; West, P. J., "Photochemical Transformations. Part XXXIII. Photolysis of Thiobenzoic Acid O-Esters. Part IV. Photolysis of O-Phenethyl Thiobenzoate Derivatives and the Formation of Thioketones," J. Chem. Soc. Perkin Trans. I 1973, 1580-1583.
5. Ogata, Y.; Takagi, K.; Ihda, S., "Type II Photoeliminations of Thiobenzoic Acid O-Esters; Photolysis of Optically Active O-2-Phenylbutyl Thiobenzoate," J. Chem. Soc. Perkin Trans. I 1975, 1725-1727.
6. Wirz, J., "Photolysis of Aromatic Thioacid O-Esters: the Nature of the Reactive Excited State," J. Chem. Soc. Perkin Trans. I 1973, 1307-1312.
7. Schrieber, A. L.; Fashing, M. A.; Abelt, C. J., "Unexpected Photoinduced Electron Transfer of Two Glucose Thiobenzoates," J. Chem. Soc., Perkin Trans 2 2000, 953-955.
8. Kavarnos, G. J.; Turro, N. J., "Photosensitization by Reversible Electron Transfer: Theories, Experimental Evidence, and Examples," Chem. Rev. 1986, 86, 401-449.
9. Lewis, F. D. Carbon-Carbon Multiple Bonds; Fox, M. A. and Chanon, M., Ed.; Elsevier: Amsterdam, 1988, pp 1-69.
10.Mattay, J., "Photoinduced Electron Transfer in Organic Synthesis," Synthesis 1989, 233- 252.
11.Mattes, S. L.; Farid, S. Photochemical Electron-Transfer Reactions of Olefins and Related Compounds, 1983; Vol. 6, pp 233-326.
12.Rao, V. P.; Rao, B. N.; Ramamurthy, V. Thiocarbonyls: Photochemical Hydrogen Abstraction Reactions; Horspool, W. M. and Song, P.-S., Ed.; CRC Press: Boca Raton, 1994, pp 793-802.
11 13.Maciejewski, A.; Steer, R. P., "The Photophysics, Physical Photochemistry, and Related Spectroscopy of Thiocarbonyls," Chem. Rev. 1993, 93, 67-98.
14.Coyle, J. D., "The Photochemistry of Thiocarbonyl Compounds," Tetrahedron 1985, 41, 5393-5425.
15.Hoshino, M.; Shizuka, H. Photoinduced Electron Transfer Reactions of Aromatic Carbonyl and Related Compounds; Fox, M. A. and Chanon, M., Ed.; Elsevier: Amsterdam, 1988, pp 313-371.
16.Alam, M. M.; Fujitsuka, M.; Watanabe, A.; Ito, O., "Laser Photolysis Study of Photochemical Reactions of Triplet States of Pyridinethiones," J. Chem. Soc. Perkin Trans. 2 1998, 817- 824.
17.Alam, M. M.; Fujitsuka, M.; Watanabe, A.; Ito, O., "Photochemical Properties of Excited Triplet State of 6H-Purine-6-thione Investigated by Laser Flash Photolysis," J. Phys. Chem. A 1998, 102, 1338-1344.
18.Alam, M. M.; Ito, O.; Adam, W.; Grimm, G. N.; Saha-Moller, C. R., "Photochemical Reactions of Triplet State of N-Hydroxyacridine-9-thione Studied by Laser Flash Photolysis," Chem. Phys., Phys. Chem. 1999, 1, 1851-1857.
19.Johnson, F. The Total Synthesis of Antibiotics; ApSimon, J., Ed.; Wiley: New York, 1973; Vol. 1, pp 337-347.
20.Sznaidman, M. Introduction to Carbohydrates; Hecht, S. M., Ed.; Oxford University Press: New York, 1999, pp 35-38.
21.Smith, J. R. L.; Masheder, D., "Amine Oxidation. Part 13. Electrochemical Oxidation of Some Substituted Tertiary Alkylamines," J. Chem. Soc. Perkin Trans 2 1977, 1732-1736.
22.Smith, J. R. L.; Masheder, D., "Amine Oxidation. Part IX. The Electrochemical Oxidation of Some Tertiary Amines: The Effect of Structure on Reactivity," J. Chem. Soc. Perkin Trans 2 1976, 47-51.
23.Lewis, F. D., "Proton-Transfer Reactions of Photogenerated Radical Ion Pairs," Acc. Chem. Res. 1986, 19, 401-405.
24.Lepley, A. R.; Closs, G. L. Chemically Induced Magnetic Polarization; Wiley: New York, 1973.
25.Roth, H. D.; Manion, M. L., "Photoreactions of ketones with amines. CIDNP criteria for the intermediacy of aminoalkyl radicals and aminium radical ions," J. Am. Chem. Soc. 1975, 97, 6886-6888.
26.Goez, M.; Sartorius, I., "Photo-CIDNP Investigation of the Deprotonation of Aminium Cations," J. Am. Chem. Soc. 1993, 115, 11123-11133.
12 27.Lide, D. R. CRC Handbook of Chemistry and Physics; 82nd ed.; CRC Press: Boca Raton, 2001, pp 5-1-60 and 9-70-72.
28.Miyokawa, K.; Tschuikow-Roux, E., "Kinetics of the Photobromination of Dichloro- and Dibromoethane. Estimate of the C-H Bond Dissociation Energies and the Heats of Formation of the CH3CCl2 and CH3CBr2 Radicals.," Bull. Chem. Soc. Jpn. 1999, 72, 1-5.
29.Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis; 3rd ed.; Wiley: New York, 1999.
30.Bordwell, F. G.; Doomes, E., "Stereochemistry and Mechanism of the Ramburg-Bäcklund Reaction. Reaction of Diastereomeric a-Halo Sulfones with Base," J. Org. Chem. 1974, 39, 2526-2531.
31.Bordwell, F. G.; Doomes, E., "Concerning the Driving Force for 1,3-Elimination Reactions. Dehydrohalogenation of 1-Halo-2-thia-2,3-dihydrophenalene 2,2-Dioxides in a Ramburg- Bäcklund Reaction.," J. Org. Chem. 1974, 39, 2531-2534.
32.Paquette, L. A.; Philips, J. C.; Wingard, J., R. E., "a-Halo Sulfones. XVIII. The Ramburg- Bäcklund Rearrangement as a Synthetic Entry to Unsaturated Propellanes," J. Am. Chem. Soc. 1971, 93, 4516-4522.
13