Walden University 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Northwestern University Feinberg School of Medicine Office of Diversity Presents
Northwestern University Feinberg School of Medicine Office of Diversity presents Friday Film Series 2012-2013: Exploring Social Justice Through Film All films begin at 12:00 pm on the Chicago campus. Due to the length of most features, we begin promptly at noon! All films screened in Daniel Hale Williams Auditorium, McGaw Pavilion. Lunch provided for attendees. September 14 – Reel Injun by Neil Diamond (Cree) http://www.reelinjunthemovie.com/site/ Reel Injun is an entertaining and insightful look at the Hollywood Indian, exploring the portrayal of North American Natives through a century of cinema. Travelling through the heartland of America and into the Canadian North, Cree filmmaker Neil Diamond looks at how the myth of “the Injun” has influenced the world’s understanding – and misunderstanding – of Natives. With clips from hundreds of classic and recent films, and candid interviews with celebrated Native and non-Native directors, writers, actors, and activists including Clint Eastwood, Robbie Robertson, Graham Greene, Adam Beach, and Zacharias Kunuk, Reel Injun traces the evolution of cinema’s depiction of Native people from the silent film era to present day. October 19 – Becoming Chaz by Fenton Bailey & Randy Barbato http://www.chazbono.net/becomingchaz.html Growing up with famous parents, constantly in the public eye would be hard for anyone. Now imagine that all those images people have seen of you are lies about how you actually felt. Chaz Bono grew up as Sonny and Cher’s adorable golden-haired daughter and felt trapped in a female shell. Becoming Chaz is a bracingly intimate portrait of a person in transition and the relationships that must evolve with him. -

Resource Material Name Author Description Copyright Year Item
Resource Material: Resource Material Copyright Item Author Description Name Year Number Guide for learning communication skills in the 10 Terrific People Skills For the New Workplace The Dartnell Corporation workplace 1997 R10001 toolkit to assist families in getting information they need in the 1st 100 days following an 100 Day Kit Autism diagnosis R10397 1001 Tips for the Parents of Autistic Girls Lyons 2010 R10426 Practical strategies to help people become better 144 Ways to Walk the Talk Erick Harvey, Alexander Lucia leaders 1997 R10002 St. Louis County Human Services Youth Programs 2015 Resources for Youth and Families - Office of Family and Community Services R10551 7 Kinds of Smart: Identifying and Developing Based on theory of "multiple intelligences," Your Multiple Intelligences Thomas Armstrong reveals the many different ways of being smart 1993 R10181 An activity-based method for evaluating and A 5 Could Make Me Lose Control Kari Dunn Buron supporting highly anxious students R10559 An activity-based method for evaluating and A 5 Could Make Me Lose Control Kari Dunn Buron supporting highly anxious students R10506 Based on the true story of a boy who compiled a book for his teachers on ways they could help A Child's Guide to Concentrating for Kids with him improve his concentration and feel more ADHD Bonita Blazer successful in school 1999 R10008 A Cup of Comfort for Parents of Children with stories that celebrate the differences in our Special Needs extraordinary kids 2009 R10382 A Framework for Understanding Poverty 1998 R10369 -

AN ANALYSIS of AUTISM THROUGH MEDIA REPRESENTATION a Lexandria Prochnow
AN ANALYSIS OF AUTISM THROUGH MEDIA REPRESENTATION A lexandria Prochnow EDITOR’S NOTE: The Next Generation series is designed to give student voices an opportunity to contribute to the ongoing conversation within the field of General Semantics and in the pages of ETC. High-school students, college undergraduates, and students in graduate programs are encouraged to submit their work. edia representation practically never accurately portrays social groups M as they actually are in reality. These social groups can be specific to certain races, ethnicities, genders, sexualities, occupations, or even medical issues; although some groups are easier and simpler to represent more truth fully, many prove difficult to depict without worrying about political cor rectness and overall accuracy. Autism, although technically a psychiatric diagnosis, forms a community of diverse people with a myriad of distinct lives and characteristics. Autistic people are a unique social group; there are some who are diagnosed that lead nearly typical lives with few challenges and others who are severely impacted and experience vast challenges. With so many different types of autism diagnoses and huge numbers of people living with autism, it would be impossible to perfectly depict each aspect of autism through television and film characters. Although not every autism characteristic or every autistic person’s story can be shown through media representation, it is media’s responsibility to at least attempt to make their portrayals as accurate as possible. Television and film are limited in what Alexandria Prochnow is a student at Fordham University in New York. Her faculty advisor on this honors thesis paper was Professor Lance Strate, a member of the IGS Board of Trustees and noted scholar in the fields of General Semantics and Media Ecology. -

11/14/13 Complete List Family & Community Resource Center
11/14/13 Complete List Family & Community Resource Center Special School District of St. Louis County 12110 Clayton Road St. Louis, MO 63131 314-989-8438/989-8108/989-8194 A+ Guide to Transitions from High School to College for Special Education. (2001/video/50 minutes) (2000/DVD) A "college prep" video for parents and students. Teachers, parents and school administrators describe the transition process and offer their best advice for having a positive experience. A is for All Aboard! Paula Kluth & Victoria Kluth (2010) Grades K and up. Fun facts, vibrant art, and in-the-know slang about trains. (32 pages) A is for Autism, F is for Friend. Joanna L. Keating-Velasco (2007) Grades 3 and up. A kid's book on making friends with a child who has autism. (54 pages) The ABA Program Companion: Organizing Quality Programs for Children with Autism and PDD. J Tyler Fovel, MA. (2002) Helps the reader integrate important theories and concepts from ABA into powerful, practical and comprehensive educational programming, from assessment through program methodology and evaluation of results. Manual & CD. The ABCs of Autism. M. Davi Kathiresan (2000) Grades K and up. This book was written to educate families, children and professionals and make them aware of the skills, strengths and capacities of persons with autism. ABCs of Emotional Behavioral Disorder. (video) (2004) (35 minutes) Outlines a best practice approach to successfully integrate elementary and middle school students with Emotional or Behavioral Disorders into the educational mainstream. ABC’s of Inclusive Child Care. (video) (1993) (14 minutes) Resource to encourage child care providers to accept children with developmental disabilities and to increase public awareness of the capabilities of individuals with disabilities. -
Library Full Listing Sorted by Title
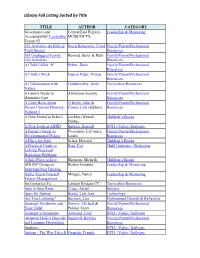
Library Full Listing Sorted by Title TITLE AUTHOR CATEGORY Governance and Central East Region - Leadership & Mentoring Accountability Leadership MCSS/MCYS Forum #2 101 Activities for Kids in Stock Kranowitz, Carol Family/Parent/Professional Tight Spaces Resources 365 Unplugged Family Bennett, Steve & Ruth Family/Parent/Professional Fun Activities Resources A Child Called “It” Pelzer, Dave Family/Parent/Professional Resources A Child’s Work Gussin Paley, Vivian Family/Parent/Professional Resources A Collaboration with Goldsworthy, Andy Curriculum Resources Nature A Family Guide to Alzheimer Society Family/Parent/Professional Dementia Care Resources A Little Book about O’Brien, John & Family/Parent/Professional Person Centred Planning - Connie Lyle (Editors) Resources Volume 1 A New Friend at School Lockrey-Wessel, Children’s Books Debbie A New Look at ADHD Barkley, Russell DVD / Video / Software A Parent’s Guide to Fivozinsky LeComer, Family/Parent/Professional Developmental Delays Laurie Resources A Place for Starr Schor, Howard Children’s Books A Practical Guide to Essa, Eva Child Guidance / Behaviour Solving Preschool Behaviour Problems A Safe Place to Live Harrison, Michelle Children’s Books ADHOC Group on Roeher Institute Leadership & Mentoring Individualized Funding Alpha Teach Yourself Mingus, Nancy Leadership & Mentoring Project Management An Invitation To…. London Bridges CC Curriculum Resources Ants in their Pants Cross, Aerial Sensory Apps for Autism Brady, Lois Jean Technology Are You Listening? Burman, Lisa Professional Growth -

Bds-Training-Events.Pdf
This is an education and training resource guide published by the NH Department of Health and Human Services Bureau ofofof Developmental Services 105 Pleasant Street Concord, New Hampshire 03301 This calendar can also be accessed through NHNHNH Department of Health & Human Services website http://www.dhhs.nh.gov/dcbcs/bds/documents/trainingcalendar.pdf October 2017 Date Time Training /Events Description Cost Location Information October 9:00 am Counseling on Suicide is the second leading $50 The Mental For more information 4 to Access to Lethal cause of death for young Health Center contact: 12:15 pm Means people ages 15 to 24 in New of Greater (C.A.L.M.) Hampshire and the tenth Manchester Joann Batty Training cause of death for those of all (MHCGM) MHCGM ages. We know that many attempters are as ambivalent Conference Phone: (603) 206-8609 Ext 5359 about suicide as they are Room about life. One piece of that Email: [email protected] puzzle that has proven to be 5 Blodget St. effective is to reduce access to Manchester, Website lethal means – particularly NH https://www.mhcgm.org/events/calm- firearms and medications. training/ This workshop addresses why and how to do this. The workshop includes: • PowerPoint presentations regarding why CALM is important • A model videotaped counseling session • Plus time for discussion and role plays. CALM is recognized on the SPRC/AFSP Best Practice Registry, Section III NH DHHS - Bureau of Developmental Services 2 October 2017 – April 201 8 Training Calen dar October 2017 Date Time Training /Events Description Cost Location Information October 8:00 am High -Functioning This intensive, full day seminar $219.99 Executive For more information 4 to Autism: provides proven intervention Single Court contact: 4:00 pm strategies, essential treatment registration Conference Proven & Practical tools, and behavioral techniques Center Pesi Inc. -

Spring 2017 M.M
EDG 666 and EDMT 621 Spring 2017 M.M. Jones College of Education & Human Services Northern Kentucky University EDMT 621: Students with Exceptionalities in the Regular Middle/Secondary Classrooms EDG 666: Introduction to the Education of Students with Exceptionalities 3 Graduate Semester Hours Credit Online ~ Spring 2017 Learn, Lead, Succeed Mission The College of Education and Human Services plays an important leadership role and collaborates with others in the creation, dissemination, and application of knowledge and research that enhances professional practice and transforms lives, schools, and communities . Vision The College of Education and Human Services aspires to be known throughout the Commonwealth of Kentucky and region at large as the leader in providing opportunities for engaged learning and applied scholarship that fosters individual growth and collective success. Kentucky Academic Standards (KAS) Preparation of Kentucky’s students for the demands of the 21st century requires districts and schools to prepare every student for successful transition to be College and Career Ready. The Kentucky Academic Standards help ensure that all students throughout Kentucky are provided with common content and have opportunities to learn at high levels. As education candidates complete and implement projects and assignments throughout their education programs at NKU, they will incorporate the components of the Kentucky Academic Standards. 1 EDG 666 and EDMT 621 Spring 2017 M.M. Jones Instructor Information Professor: Dr. Missy Jones Office Location: MEP 286 Office Telephone Number: (859) 572-1423 FAX: 859-572-6096 e-mail: [email protected] Office Hours: Wednesdays 11:00 a.m. -2:00 p.m. Thursdays 1:00-4:00 p.m. -

Kitchener Public Library - Autism Dvds, VHS, Cds, and Multimedia Kits Total Collection As of March 2008
Kitchener Public Library - Autism DVDs, VHS, CDs, and Multimedia Kits Total Collection as of March 2008 Title Date All about Jesus [multimedia] 1999 Andrew's plan [videorecording] 2000 Animals in translation [compact disc] : using the mysteries of autism to decode animal behavior / Temple Grandin and Catherine Johnson 2005 Asperger syndrome [videorecording] : living outside the bell curve / Video Architects Attainment Company. -- 2001 Asperger's syndrome [videorecording] / Tony Attwood 1999 Asperger's syndrome [videorecording] / Tony Attwood 1999 Asperger's syndrome. Volume 2 [DVD] / Tony Attwood 2003 Autism [videorecording] / Mystique Films, in association with Discovery Health Channel Canada produced by Christian Bruyère, Mary Bissell created by Mary Bissell directed and written by Christian Bruyère. -- 2004 Autism: a world apart [videorecording] / Films for the Humanities & Sciences 1995 Autism and applied behavioral analysis [videorecording] / ABC News. -- 2001 Autism awareness video for law enforcement [videorecording] / by Dennis Debbaudt Autism Society Ontario, Waterloo County Chapter 2002 The autism epidemic? [sound recording] : an international perspective / J. Holden, ... [et al.] Unknown Autism is a world [DVD] / a co-production of CNN Productions and State of the Art, Inc. ... producer and director, Gerardine Wurzburg writer, Sue Rubin 2005 Autism [videorecording] : neurological research & neuro-developmental therapy / produced by Svea J. Gold. -- 2000 Autism spectrum disorders Attainment Company produced by Glenis Benson directed by Sue and Dick Geir. -- 2002 Autism spectrum disorders & the SCERTS model [videorecording] : a comprehensive educational approach / National Professional Resources executive producer, Robert M. Hanson associate producer, Nancy R. Cassone. -- 2004 Autism [videorecording] : the road back / produced by Knowledge Network 2003 Autism -- there's hope out there [DVD] / Thinking Hat Productions directed by Jim Reed 2006 Barbara & Sheldon's story [videorecording] : living with autism / produced by Vital Signs, Inc. -

Prince William County Public Schools Office of Special Education Parent
Prince William County Public Schools Office of Special Education Parent Resource Center Lending Library Bibliography 1 LIBRARY CONTENTS Place the cursor on the underlined words for the category you want. Hold down the Control key (CTRL) and click the mouse. To return to this page, hold CTRL and click on the link at the end of each section. GENERAL SPECIFIC DISABILITIES General (topics cross multiple categories) Attention Deficit [Hyperactive] Disorder Family Autism Inclusion Emotional Disability Legal Hearing Impairment Parenting (Behavior) Intellectual Disability Sex Education Learning Disabiliy Social Skills/Self-Esteem Orthopedic Impairment Transition Adult Other Health Impairment Preschool Speech and Language Traumatic Brain Injury Visual Impairment Reference Children’s Books Parents are welcome to use these resources within Located in each specific disability, are books the Parent Resource Center. They cannot be written for children. Many are useful for disability checked out. awareness. On the library shelf, these books have an orange strip with a penguin at the top of the spine. Non-Print Media Education Professional Materials such as, DVD’s, Audio Tapes, VHS Materials designed for school staff. Many may also Video’s, and CD’s, are housed within each specific be of help to parents. disability. Many are useful for disability awareness. Autism Processing Behavior Reading General Skills Language Test Learning Disability Transition 2 NEW SECTION EDUCATION PROFESSIONAL AUTISM Buron, Kari Dunn and Curtis, Mitzi. The Incredible 5-Point Scale: Assisting Students with Autism Spectrum Disorders in Understanding Social Interactions and Controlling Their Emotional Responses. Shawnee Mission, KS: Autism Asperger Publishing Company, 2003. Coyne, Phyllis, Nyberg, Colleen, and Vandenburg, Mary Lou. -

Autismus Therapie Zentrum Niederrhein
Autismus: Literatur, Medien, Internet usw. Stand: 17.04.2017 09:11 Seite 1 von 58 (www.autismus-online.de) + Autismus-Inhouse-Fortbildungen © Harald Matoni Autismus Literatur, Materialien, Internet usw. Inhaltsverzeichnis: 1. Autismus-Spektrum-Störungen (ASS), Autismus (Frühkindlicher Autismus / Kanner-Syndrome, Atypischer Autismus, …) + tiefgreifende Entwicklungsstörungen ..................................................................................... 3 2. Asperger-Syndrom usw. ................................................................................................................................. 7 3. Autismus und Asperger-Syndrom - einfach beschrieben - leicht verständliche Empfehlungen – Anleitungen für Betroffene – kleine Geschichten .............................................................................................................. 10 3.1 Material von Claudio Castaneda u.a. ............................................................................................................. 11 4. Autismus – Das „Anders-Sein“ erarbeiten .................................................................................................... 11 5. Autismus, Asperger-Syndrom, Behinderung – für Geschwister, Freunde, Mitschüler und andere ................. 12 6. Rett-Syndrom ................................................................................................................................................ 12 7. „Klassiker“ .................................................................................................................................................. -

Autism Speaks School Community
About this Kit As the rate of autism diagnosis increases, many more public and private schools will include students with autism. Learners with autism may have some additional challenges in the school environment, but with the support of the school community – the teachers, administrators, aides, office staff, bus drivers, nurses, custodians, peers and parents -- students with autism can make great strides and become valued members of a student body. Just as students can learn from each member of the school community, the school staff and peers can learn that students with autism have a lot to offer in return. The purpose of this kit is to provide information about autism – the features, challenges and strengths -- as well as some of the tools and strategies that may result in more positive interactions for all members of a school community. This tool kit is not intended to be a curriculum for special education for students on the autism spectrum, but rather a support for the general education and administrative school staff who interact with students with autism in various capacities. However, it is envisioned that this tool kit will provide valuable information and resources that can be employed by special education and administrative staff in their efforts to plan for and support students in general education environments and involvement in the school community as a whole. The following information has been compiled to assist in staff training efforts, offering an introduction to autism and highlights of specific strategies that have been found to be helpful. It is important that support for students with autism employs a team approach, and that each student is considered on an individualized level, in addition to the general perspective that is provided here. -

Film Industry Portrayals of Autism Spectrum Conditions and Their Influences on Preservice Teachers in Australia Andrea Roxanne Garner University of Wollongong
University of Wollongong Research Online University of Wollongong Thesis Collection University of Wollongong Thesis Collections 2014 What’s showing: film industry portrayals of autism spectrum conditions and their influences on preservice teachers in Australia Andrea Roxanne Garner University of Wollongong Recommended Citation Garner, Andrea Roxanne, What’s showing: film industry portrayals of autism spectrum conditions and their influences on preservice teachers in Australia, Doctor of Philosophy thesis, , University of Wollongong, 2014. http://ro.uow.edu.au/theses/4282 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] What’s Showing: Film Industry Portrayals of Autism Spectrum Conditions and their Influences on Preservice Teachers in Australia A thesis submitted in fulfilment of the requirements for the award of the Degree of Doctor of Philosophy from the University of Wollongong by Andrea Roxanne Garner MEd, PGDE, BA November 2014 Declaration I declare that this thesis is wholly my own work unless otherwise referenced and acknowledged. The document has not been submitted for qualifications at any other academic institution. Andrea Roxanne Garner 27 November 2014 i Acknowledgements Many thanks are owed to my supervisors: Professor Sandra Jones and Associate Professor Valerie Harwood. Without their time, patience, persistence, and guidance I would not have been able to produce this body of work. Additionally, I would like to offer a special mention to Christine Carey who shared her time and expertise as the second rater in the film analysis, and Noelene Wetherby-Fell who championed the engagement of the participants for this thesis.