Stoichiometry Tutorial Name ______Period ___
*Selected answers are at the end of this tutorial, but remember not to peek unless you REALLY REALLY REALLY REALLY need to.
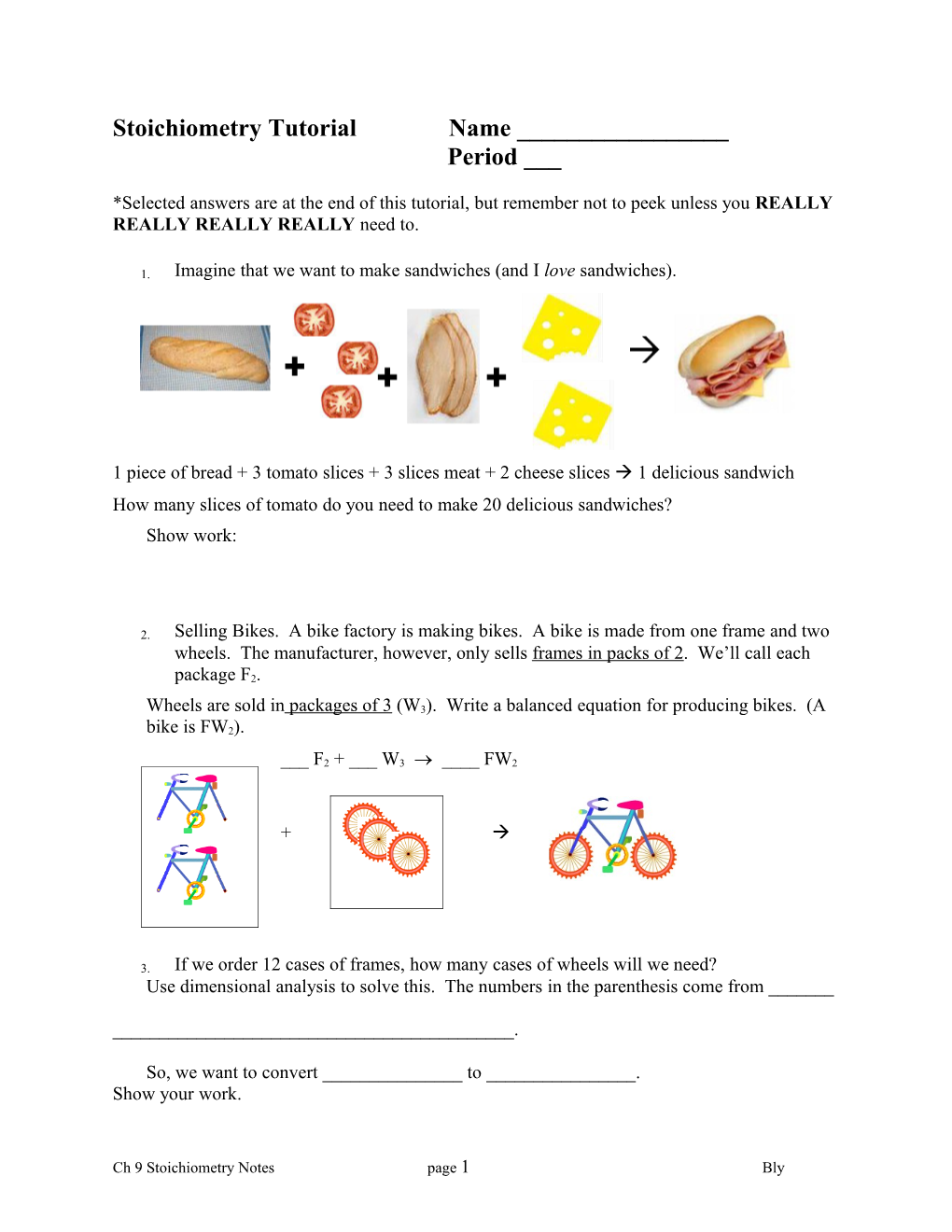
1. Imagine that we want to make sandwiches (and I love sandwiches).
1 piece of bread + 3 tomato slices + 3 slices meat + 2 cheese slices 1 delicious sandwich How many slices of tomato do you need to make 20 delicious sandwiches? Show work:
2. Selling Bikes. A bike factory is making bikes. A bike is made from one frame and two wheels. The manufacturer, however, only sells frames in packs of 2. We’ll call each package F2.
Wheels are sold in packages of 3 (W3). Write a balanced equation for producing bikes. (A bike is FW2).
___ F2 + ___ W3 ____ FW2
+
3. If we order 12 cases of frames, how many cases of wheels will we need? Use dimensional analysis to solve this. The numbers in the parenthesis come from ______
______.
So, we want to convert ______to ______. Show your work.
Ch 9 Stoichiometry Notes page 1 Bly The coefficients can represent
the number of molecules and atoms OR
The moles of molecules and atoms
N2(g) + 3H2(g) 2NH3(g)
1 molecule N2 + 3 molecules H2 2 molecules NH3
1 mol N2 + 3 mol H2 2 mol NH3
4. Mole Ratio: The ratio between two chemicals in a balanced chemical equation. It is used as a conversion factor. For example, balance this reaction.
____ Fe + ____ O2 ____ Fe2O3
5. The mole ratio for O2 to Fe2O3 is ___ mol O2 : ___ mol Fe2O3
6. How many moles of O2 are needed to form 21.0 moles of Fe2O3? (Convert 21.0 mol Fe2O3 to mol O2)
21.0 mol Fe2O3 ______=
7. Stoichiometry (stoik ee ahm ih tree)Using mole ratios to convert from an amount of one substance to the amount of another substance If you know the amount of chemical A and you want to know the amount of chemical B, then it is just converting moles of A to moles of B using the mole ratio.
moles A moles B mole ratio
8. Questions may ask for the answer as a mass (in grams) rather than moles. You can think of a chemical equation as number of molecules, moles AND grams N2 (g) + 3H2 (g) 2NH3 (g) 1 molecule N2 + 3 molecules H2 2 molecules NH3 1 mol N2 + 3 mol H2 2 mol NH3
Ch 9 Stoichiometry Notes page 2 Bly 28 g N2 + 3 x 2.02 g H2 2 x 17g NH3
Ch 9 Stoichiometry Notes page 3 Bly N2 (g) + 3H2 (g) 2NH3 (g)
• How many grams of NH3 can be made from 38.4 grams H2?
N2 (g) + 3H2 (g) 2NH3 (g)
• The equation only gives the “mole” ratio, so we need to convert grams to moles. • Plan: Grams H2 moles H2 moles NH3 grams NH3
9. How many grams of Fe2O3 are formed when 6.00 moles of O2 react?
a. Convert ______to ______
b. Still use the mole ratio, but then convert mol Fe2O3 to g Fe2O3 a separate step.
6.00 mol O2 mol Fe2O3 ______mol O2
moles A moles B grams B mole ratio molar mass
10. Or, the question could start with the mass of the given chemical. What mass (in grams) of Fe is needed to react with 525 g of O2?
grams A moles A moles B grams B molar mass mole ratio molar mass
Show work here.
Ch 9 Stoichiometry Notes page 4 Bly 11. Are you sick of stoichiometry yet? If so… Milk of Magnesia, which contains magnesium hydroxide, reacts with stomach acid (HCl) to neutralize it. a. Write the formulas for the two reactants to the left of the arrow below.
+ +
b. Which type of reaction could this be? ______
c. Write the formulas for the two products, and balance the equation in part “a.”
d. How many moles of water will be produced if 3.00 mol magnesium hydroxide react?
e. How many grams of the other product (not water) will be produced if 3.00 g of magnesium hydroxide reacts?
f. What mass of HCl is required to completely react with 3.00 g of magnesium hydroxide?
Ch 9 Stoichiometry Notes page 5 Bly Answers to selected problems 2. 3, 4, 6 3. Convert cases of frames (F2) to cases of wheels (W3) a. What you know is 12 cases of frames. So, 12 F2 b. Units to cancel are F2, so put that on the bottom of the fraction. c. You want to get cases of wheels, W3 d. 16 W3
6. 31.5 mol O2
9. a. Convert 6.00 mol O2 to g Fe2O3 b. Molar mass of Fe2O3 is 159.70 g/mol. So answer is 639 g Fe2O3. (3 sig figs because 6.00 mol) Man, I love sig figs!!
11. Mg(OH)2 + 2 HCl MgCl2 + 2 H2O
Ch 9 Stoichiometry Notes page 6 Bly Section 9.3 Limiting Reactants More sandwiches (yum, sandwiches…)
1 piece of bread + 3 tomato slices + 3 slices meat + 2 cheese slices 1 delicious sandwich We have: 20 pieces of bread, 44 slices of tomatoes, 332 slices of meat and 4 slices of cheese. o How many sandwiches can you make? ______o Show work:
We need to buy more ______if we want to make more, and I want to watch the soccer game, so I can’t go to the store right now. In the sandwich example, cheese was the limiting reactant (also called “limiting reagent). o The limiting reactant is completely used up in a reaction.
Selling Bikes F = frame, W = wheel Wheels come in packages of 3, Frames come in packages of 2 3 packages of frames and 4 packages of wheels make six bikes:
3 F2 + 4 W3 ® 6 FW2 We have 21 cases of frames left in storage, but no wheels. How many packages of wheels must we buy? (Show work!)
Oops. Actually, I just found 24 packages of wheels under my desk. So, do we use up the wheels, or do we have extra wheels? ______ What is the limiting reactant? ______
Ch 9 Stoichiometry Notes page 7 Bly Determining the Limiting Reactant
N2 (g) + 3H2 (g) 2NH3 (g)
To use all 16.0 mol N2, you would need 48.0 mol H2.
So what if you had 16.0 mol N2 and 19.0 mol H2. Which would you use up of first? ___
You use up the limiting reactant. The chemical that you have too much of is the excess reagent. Using the Limiting Reactant
You have 16.0 mol N2 and 19.0 mol H2. How many moles of NH3 will form? Which will determine how much product forms—the limiting reactant or the excess reactant? ______ Since limiting reactant controls the amount of product formed, just convert from
19.0 mol H2 to mol NH3.
How many moles of the excess reactant would remain?
Example: 25.0 kg N2 react with 5.00 kg H2. How much ammonia (NH3) will be made?
N2 (g) + 3H2 (g) 2NH3 (g)
1. Calculate moles of H2 and N2.
3. Ask yourself: Do you have enough H2?
Yes H2 is excess, N2 is limiting.
No H2 is limiting, N2 is excess
4. Use the limiting reactant to calculate grams NH3.
Ch 9 Stoichiometry Notes page 8 Bly Tin(II) fluoride is used in some toothpastes.It is made by reacting tin with hydrogen fluoride to produce tin(II) fluoride and hydrogen gas. a. Write a balanced chemical equation for this reaction. b. You have 3.15 mol of Sn and 4.96 mol HF. i. Which is the limiting reactant? (show work!)
ii. How many moles of tin(II) fluoride will form?
c. You have 21.1 g Sn and 84.5 g HF. i. Which will be limiting? ______ii. What mass of tin(II) fluoride will form?
Percent Yield In a real experiment, usually you get less product than you would expect. Sometimes not all the chemicals react. Theoretical Yield – calculated amount of product Actual Yield – amount of product actually collected in lab Example Knowing both of these, you can calculate percent yield. % yield = actual yield ´ 100% theoretical yield In an experiment, Jenny collected 57.1 g of product. However, stiochiometrically she calculated that she should have gotten 66.8 g. Find the percent yield. 57.1 g ´ 100% = 85.5% yield 66.8 g
Ch 9 Stoichiometry Notes page 9 Bly Key
p. 7 How much NH3 will form when 25.0 kg of N2 react with 5.00 kg H2? 1. 892 mol N2, 2480 mol H2
2. You need 827 mol N2 to react with 2480 mol H2 3. H2 is the limiting reactant 4. 28200 g NH3 p. 8 Part 1 b. i. HF is limiting ii. 2.48 mol SnF2 c. i. Sn is the limiting reagent, 0.178 mol Sn, 4.45 mol HF ii. 27.9 g SnF2
Ch 9 Stoichiometry Notes page 10 Bly