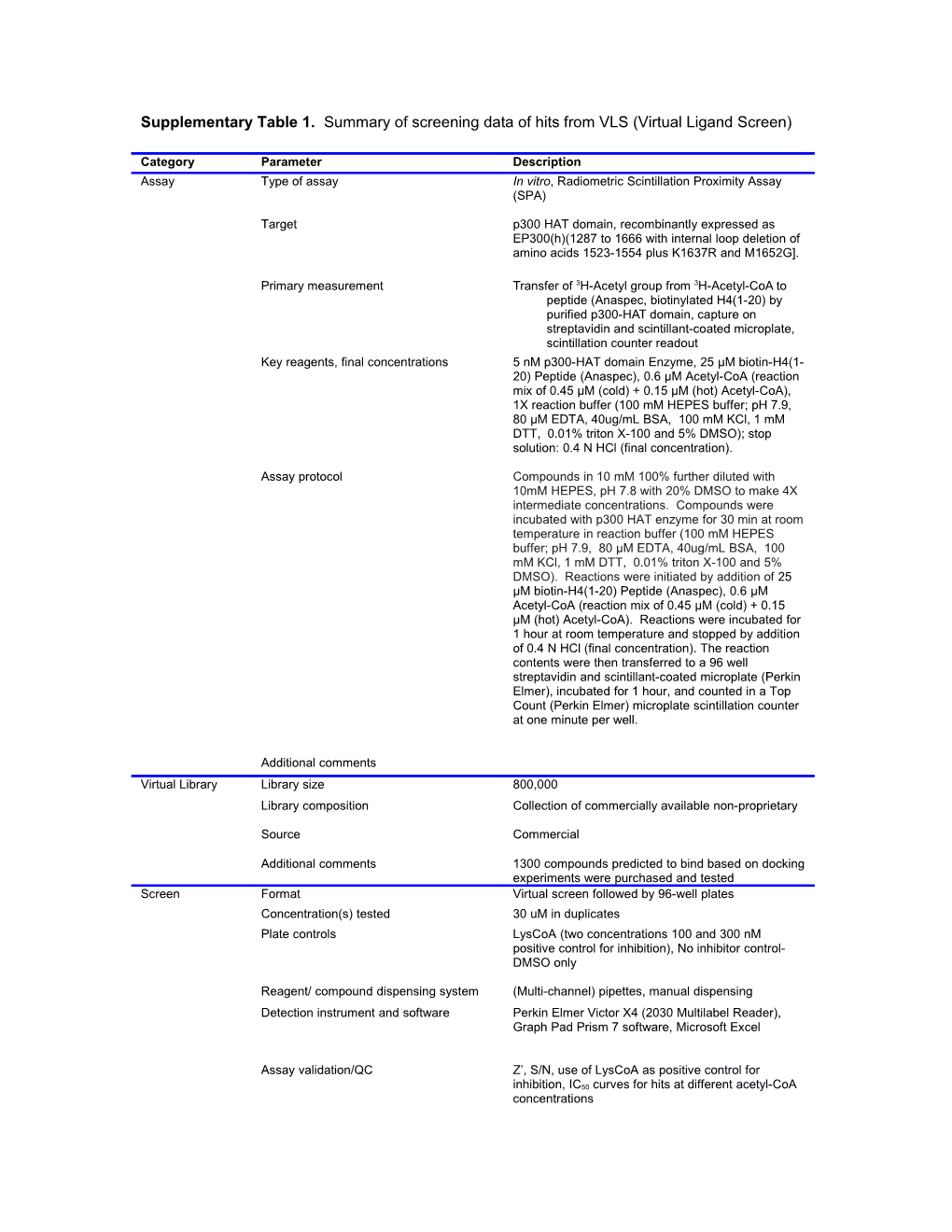
Supplementary Table 1. Summary of screening data of hits from VLS (Virtual Ligand Screen)
Category Parameter Description Assay Type of assay In vitro, Radiometric Scintillation Proximity Assay (SPA)
Target p300 HAT domain, recombinantly expressed as EP300(h)(1287 to 1666 with internal loop deletion of amino acids 1523-1554 plus K1637R and M1652G].
Primary measurement Transfer of 3H-Acetyl group from 3H-Acetyl-CoA to peptide (Anaspec, biotinylated H4(1-20) by purified p300-HAT domain, capture on streptavidin and scintillant-coated microplate, scintillation counter readout Key reagents, final concentrations 5 nM p300-HAT domain Enzyme, 25 µM biotin-H4(1- 20) Peptide (Anaspec), 0.6 µM Acetyl-CoA (reaction mix of 0.45 µM (cold) + 0.15 µM (hot) Acetyl-CoA), 1X reaction buffer (100 mM HEPES buffer; pH 7.9, 80 µM EDTA, 40ug/mL BSA, 100 mM KCl, 1 mM DTT, 0.01% triton X-100 and 5% DMSO); stop solution: 0.4 N HCl (final concentration).
Assay protocol Compounds in 10 mM 100% further diluted with 10mM HEPES, pH 7.8 with 20% DMSO to make 4X intermediate concentrations. Compounds were incubated with p300 HAT enzyme for 30 min at room temperature in reaction buffer (100 mM HEPES buffer; pH 7.9, 80 µM EDTA, 40ug/mL BSA, 100 mM KCl, 1 mM DTT, 0.01% triton X-100 and 5% DMSO). Reactions were initiated by addition of 25 µM biotin-H4(1-20) Peptide (Anaspec), 0.6 µM Acetyl-CoA (reaction mix of 0.45 µM (cold) + 0.15 µM (hot) Acetyl-CoA). Reactions were incubated for 1 hour at room temperature and stopped by addition of 0.4 N HCl (final concentration). The reaction contents were then transferred to a 96 well streptavidin and scintillant-coated microplate (Perkin Elmer), incubated for 1 hour, and counted in a Top Count (Perkin Elmer) microplate scintillation counter at one minute per well.
Additional comments Virtual Library Library size 800,000 Library composition Collection of commercially available non-proprietary
Source Commercial
Additional comments 1300 compounds predicted to bind based on docking experiments were purchased and tested Screen Format Virtual screen followed by 96-well plates Concentration(s) tested 30 uM in duplicates Plate controls LysCoA (two concentrations 100 and 300 nM positive control for inhibition), No inhibitor control- DMSO only
Reagent/ compound dispensing system (Multi-channel) pipettes, manual dispensing Detection instrument and software Perkin Elmer Victor X4 (2030 Multilabel Reader), Graph Pad Prism 7 software, Microsoft Excel
Assay validation/QC Z’, S/N, use of LysCoA as positive control for inhibition, IC50 curves for hits at different acetyl-CoA concentrations Correction factors None
Normalization Data reported as % control (no inhibitor-DMSO only)
Additional comments Post-HTS analysis Hit criteria >30% inhibition at 30 uM
Hit rate 2 out of 1,700 compounds tested = 0.1%
Additional assay(s) Further confirmation assays described in detail in this manuscript.
Confirmation of hit purity and structure Compounds were verified for identity by mass spectrometry and NMR spectroscopy. Purity was established by HPLC with UV detection at 220 and 254 nM. Compounds were later resynthesized for confirmation.
Additional comments N/A